Soil pollution is a major environmental problem due to irrational human activities. Electro-bioremediation of contaminated soils is an emerging hybrid technology, which combines the advantages of electrokinetics and bioremediation in the treatment of polluted soils, namely efficient, sustainable and low-cost. However, the extreme pH variations in the soils, caused by the electrokinetical and electrochemical processes using direct current field (DC field), are the main drawback and have a detrimental effect on microorganisms in the electro-bioremediation technology. This study applied alternating current field (AC filed) with 50 Hz frequency in electrokinetics to successfully maintain the pH at neutral range and evaluated the resulting influences in the biodegeration of petroleum hydrocarbons in contaminated soils. The maximum voltage gradient of AC field was 0.5 V/cm between two electrodes in the electrokinetics and the mixed microorganisms obtained from oil sludge were utilized to decompose the contaminants in the bioremediation process. Although the microbial counts decreased slightly under AC field due to the electrical stimulation, the removal efficiency of pollutants in 21-day long electro-bioremediation reached up to 31.6%, which was improved more than 2 times as compared to the single bioremediation process. The removal of contaminants by single electrokinetics using AC field was negligible, indicated that electrokinetic process played a more important role than the electrochemical process in electro-bioremediation. AC field could induce reciprocating electrokinetic movements of various substances in the soil to enhance mass transfer at the micro scale, resulting the frequent interactions between the microbial and other substances like pollutants and nutrients. Therefore, applying AC field in electrokinetics is a promising stratagem to control the pH for microbial growth and promote bioremediation for the remediation of petroleum-contaminated soils.
A rapid growth in the consumption of plastic can be observed all over the world, which has led to huge quantities of accumulated plastic waste. The untreated plastic waste has numerous negative implications on the environment and up to now, its recycling mainly uses traditional two-step process including sorting and melting, which requires high energy input. In this work, we developed a novel and facile method to transform the plastic waste into one type of material with high surface area. It simply involved the dissolution of the plastic waste and further the fast crosslinking under certain reaction conditions. Reaction conditions such as solvent, reaction temperature and substrate ratio were shown to affect the structure of the material. It was discovered that the optimal material possessed a high surface area with around 1000 m2/g and its pore size distribution (PSD) was mainly in the microporous range. In terms of its application in dye removal, the obtained material had a comparable even better performance with other adsorption materials such as metal organic frameworks (MOFs) and polymers synthesized from expensive fine substrates. This work simultaneously recycled the environmental unfriendly plastic waste and reused it into solving another crucial environmental issue.
Two kinds of clay particles; bentonite and kaolin were spray dried after treated with various pH conditions ranging from pH 2 to pH 10. SEM image of the spray dry-treated bentonite showed more spherical morphology compared to kaolin. Bentonite with high mineral composition of 2:1 clay montmorillonite, have structure of tetrahedral-octahedral-tetrahedral (TOT) coordination allowing high adsorption capacity of cation compared to 1:1 clay kaolin. In this study, the dried clay particles were subjected to the ammonium solution and their adsorptive capacity was measured in term of zeta potential values. During the treatment, both clay particles indicate decreasing zeta potential values when the pH values were varied from acidic condition in pH 2 to basic condition in pH 10. Bentonite and kaolin were indicated with -8.46 mV and +9.83 mV in the initial acidic condition and the value decrease to -15.93 mV and -40.77 mV respectively in the final basic condition. The adsorption capacity of treated bentonite and kaolin were conducted with ammonium solution for 5 days of contact time. The magnitude of zeta potential values of pH 2-treated particles were increasing for bentonite and decreasing for kaolin. When the pH was higher than pH 2, both particles showed decreasing zeta potential values until they reach a plateau of ~ -15 to -20 mV for bentonite and ~ -20 to -35 mV for kaolin.
Sewage sludge ash, which is sampled from N&S city central wastewater treatment plant in Japan, is contained about 30% (W/W) phosphorus. This content could be a potential phosphorus resource. In this study, a new recovery method including acid & alkali dissolution and precipitation processes was proposed for recovering phosphorus and removing heavy metals from sewage sludge ash. This method could achieve a high phosphorus recovery rate from sewage sludge ash and gained the final product that could be directly using as phosphate fertilizer. The influencing factors such as reaction time, reaction temperature, liquid to solid ratio and pH have been examined. The optimum conditions of acid dissolution have been obtained, also, a liquid to solid ratio and reaction time discussed. Obtained phosphorus recovery rate was very high approximately twice as big as traditional alkali process. In the optimum conditions, average recoveries of above 80% of phosphorus were obtained as well as a high heavy metals removal rate that guaranteed their content of final product to meet the national standard of fertilizer.
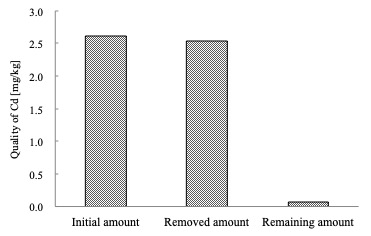
Precipitation rate of heavy metal increased as increasing pH generally at acid process. In alkali process, their dissolution rate decreased as increasing pH of precipitation. These results gave a good removal of heavy metals from sewage sludge ash. For example, Fig. 1 shows the mass balance of Cd quality over recovery processes, which illustrates that final remaining amount of Cd was far below initial amount of sewage sludge ash. This result proves that present experiment method is available for removal of heavy metal.
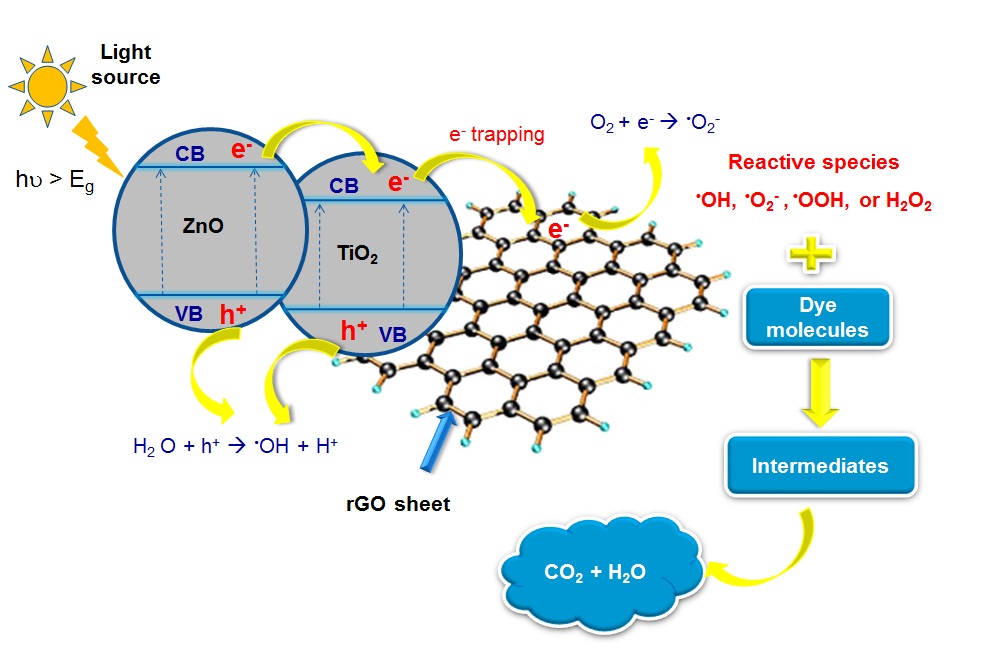
In this study, the TiO2/ZnO/rGO (TZR) composite was fabricated by a facile hydrothermal method. The X-ray diffraction and Raman spectra confirmed the formation of wurtzite hexagonal ZnO, anatase TiO2, and reduced graphene oxide (rGO) in the composite. The UV-visible diffuse spectra indicated that the composite exhibited a broad light absorption in a longer wavelength region and reduced bandgap energy with a lowest value of 2.7 eV. Photoluminescence and photocurrent measurements also demonstrated more efficient separation of photogenerated electron-hole pairs of TZR composites than ZnO, P25 TiO2, and pure TiO2/ZnO, attributing to chemical interactions between TiO2, ZnO, and rGO. Photocatalytic activity of the catalysts was evaluated on the degradation of three model dyes (methylene blue, rhodamine B, and methyl orange) under UV and simulated solar light irradiation. The synergistic effect of ternary components in the composite (TiO2, ZnO, rGO) led to a higher photocatalytic activity than ZnO and TiO2/ZnO. The varying loadings of graphene oxide (GO) in the precursor revealed a significant effect on the characteristics of TZR composites. Notably, the photocatalytic performance of a TZR composite with 5 wt% GO and an equal molar ratio of TiO2 and ZnO (denoted as TZR5) was better than the benchmarking catalyst P25 on the degradation of methylene blue and rhodamine B. The highest decolorization and mineralization of 99.2% and 41.1% was achieved, respectively, for methylene blue after 120-min UV irradiation; moreover, they were 98.2% and 48.0% for rhodamine B as well as of 98.4% and 44.2% for methyl orange after 180-min UV exposure. Under simulated solar light irradiation, photocatalytic degradation of three dyes over TZR5 also was more efficient than that over P25, ZnO, and TiO2/ZnO.