
Climate change effects that scientists had predicted in the past are now occurring: loss of sea ice, accelerated sea level rise, more frequent and more intense heat waves, drought in some regions, as well as tropical storms. Besides the direct damages to the ecosystems by wildfires, more carbon dioxide (CO2) is released in the atmosphere. As a greenhouse gas, CO2 traps heat in the Earth's lower atmosphere which in turn causes the rise in global temperatures.
At the 2015 Paris Agreement, the world leaders pledged to keep a global temperature rise this century to well below 2 degrees Celsius above pre-industrial levels. However, in Oct 2018, World Meteorological Organization (WMO) reported that the average global surface temperature in 2018 was already 0.98 degrees Celsius above the levels. Meanwhile, NASA scientists reported that the warming in the last 60 years is attributed to human activities.
The oral presentation will cover on the following aspects:
· The challenges and opportunities in tackling climate change issues for industries.
· Preparation in terms of climate change mitigation and adaption strategy
· Human capital that a country need to have and invest in in order to benefit from available opportunities.
· Specific opportunities for oil & gas industries that should be capitalized.
Many end-of-life products, such as electronic goods and catalytic converters in cars, are important sources of precious and rare metals. Although conventional thermal and chemical recycling techniques remain the best methods for recycling precious and rare metals, these metals have yet to be fully utilized. Therefore, further research and development is needed to fully recycle precious and rare metals from secondary sources.
We believe that biological technologies now provide an attractive and eco-friendly alternative strategy. This paper describes our research results from using new biotechnologies to fully recycle platinum group metals (PGMs) from spent automotive catalysts and gold from electronic waste. We focused on using the metal ion-reducing bacterium, Shewanella algae, to recover PGMs from the aqua regia leachate of spent automotive catalysts. The Shewanella bacteria were able to reduce aqueous PGMs ions (Pd(II), Pt(IV) and Rh(III)) in the catalyst leachate as metallic nanoparticles on the bacterial cells at room temperature and pH 6 within 60 min, using formate as the electron donor. We also employed baker's yeast, Saccharomyces cerevisiae, as a commercially available biomaterial for collecting gold ions from the aqua regia leachate of spent central processing units (CPUs). The baker's yeast was able to rapidly and effectively collect only gold ions from the CPU leachate at pH 1 within 10 min. Importantly, baker's yeast did not react with other heavy metal ions, such as copper, nickel, and iron.
Unlike conventional recycling processes, the benefits of our new bioprocess include a significant reduction in energy consumption and material consumption, and a low environmental impact. Our highly efficient bioprocess could be introduced at local collection points for end-of-life products and operate as a regionally distributed technology for fully recycling metal resources from end-of-life products, which will lead to the sustainable use of PGMs and gold.
Abstract: Many extraction systems are required to be completed at a high phase ratio (>20:1). However, operation at high phase ratio causes some issues including low specific surface area, high mass transfer resistance and long mass transfer distance, leading to the long residence time or low extraction efficiency. In this paper, gas-liquid-liquid (G/L/L) micro-dispersion technology is used to intensify the mass transfer and the extraction of mixed rare earth elements (REEs). The results of liquid-liquid system show that the extraction efficiency decreases as the phase ratio increases, which further reflects the challenge of extraction at high phase ratio. Subsequently, the gas phase is used to enhance the mass transfer, and the overall mass transfer coefficient is used to characterize the mass transfer rate quantitatively. The results show that the overall mass transfer coefficient after gas introduction can be up to 8 times as high as that of the non-gas introduction, which demonstrates that the introduction of gas phase greatly accelerates the mass transfer and achieves the enrichment and recovery of rare earth ions successfully. In addition, the extraction of medium and heavy REEs mixtures are also intensified by this method, which proves the universality and versatility of this method.
Cobalt (Co) is a key element in the cathode material of lithium–ion batteries (LIBs). With the rise in LIBs applications, Co demand continued to grow strongly. However, shortage of global Co supply is imminent. Hydrometallurgical is usually used to recycling Co from spent LIBs. The objective of the study is to separate Co from Li, by flotation method in mixed solution, which simulates the solutions generated in the recycling of spent LIBs. When an anionic surfactant, such as sodium dodecyl sulfate (SDS) was used as the collector, ca. 98% of Co ions was separated from the solution. It is due to its adsorption via electrostatic interaction with SDS. It was found that Li ions were hardly separated because of the weak of interaction with anionic collector. Flotation process may follow different flotation mechanisms under various pH values. In this study, ion flotation occurred at pH 4.5, 5.5, and 7.5 and precipitate flotation occurred at pH 9 when Co formed cobalt hydroxide precipitate (Ca(OH)2). Separation efficiency of Co in the solution was affected by surfactant types, concentration of surfactant, pH, and N2 gas flow rate. Various types of anionic surfactants were used as collector and compared. Structure of anionic surfactant influenced significantly the separation efficiency of Co in the flotation experiment.
Keywords: Cobalt, flotation, lithium, separation, surfactant.
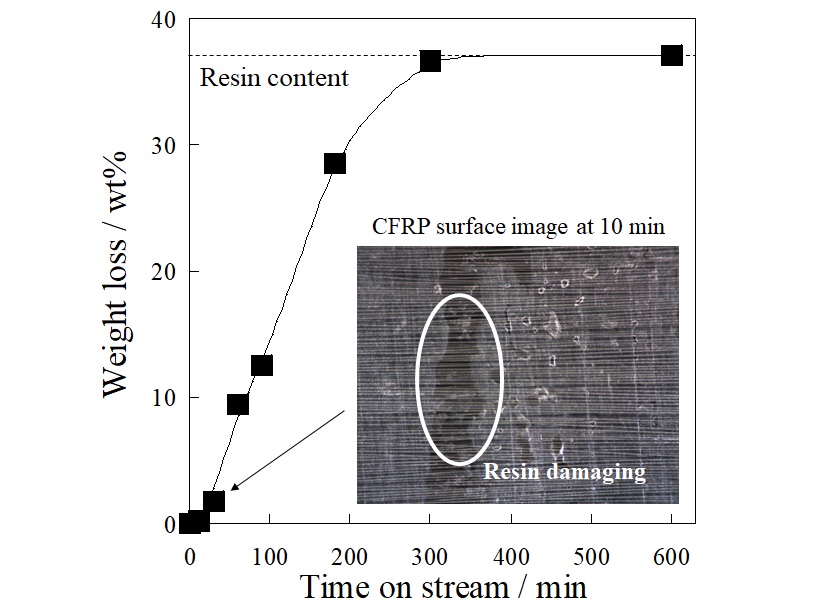
Carbon fiber reinforced plastic (CFRP) is a composite material of carbon fiber and resin, and it has properties of light weight and high strength, so its demand is expected to increase in the future. To recycle carbon fiber, which is an expensive material, is desirable, but its recycling method has not been established. The most effective method is thermal pyrolysis, but the thermal damage of carbon fibers is difficult to avoid. Therefore, we focused on a resin separation from CFRP by an electrical treatment. We have found that the electrical treatment can separate the resin from CFRP laminates in our previous research, but the details of the mechanism have not been revealed. In this paper, we applied the electrical treatment on a unidirectional CFRP mono-layer which was easy to discuss the mechanism and investigated the separation mechanism. The electrical treatment was performed using a two-electrode cell with CFRP mono-layer as an anode. The applied voltage was 15 V at constant, and the electrolyte was neutral solution. As shown in Figure, the weight loss of CFRP mono-layer reduced with time on stream, and it reached at the resin content at 300 min. The result indicated that the resin can be separated from the CFRP mono-layer in 300 min by the electrical treatment. From the digital microscope observation, the resin damage on CFRP mono-layer was observed at 10 min after the treatment. In addition, no resin component dissolved in the electrolyte was detected from various analysis. Furthermore, only water electrolysis proceeded during the voltage application. From these results, it was suggested that the resin separation by electrical treatment proceeds by the following mechanism; the water electrolysis proceeds on the carbon fiber, and the gas generated by the electrolysis peels off the resin adhering to the surface.
Plastics are raw materials of various molding products including heat-stable boards and IC devices. Recycling of plastic wastes has been interested from the point of view of resources and the environment, however, mechanical recycling of plastic wastes of such as thermo setting resin is not easy because they do not melt and mechanical recycling can not be applied. In this study, IC packages and foamed phenol resin were treated in high temperature solvents. Separation of organic materials and inorganic parts was attained in the case of the IC package by the thermal treatment in high temperature water and following solvent extraction. Foamed phenol resin was solubilized by the treatment in organic solvent at relatively mild temperature, and the production of monomeric materials such as phenol and cresols were confirmed. The presence of chemical participation of the solvent to decompose the methylene bonds contained in thermo setting resin was also confirmed in the reaction at 400oC. Such reaction mechanism was also suggested by the reaction of model compounds of thermo setting resin in high temperature solvent. The effective decomposition reaction show that solvents play important roles not only hydrogen donor, but also physical and solvolysis reactants. Plastic materials were decomposed also at relatively low temperature at 300 to 350oC.