
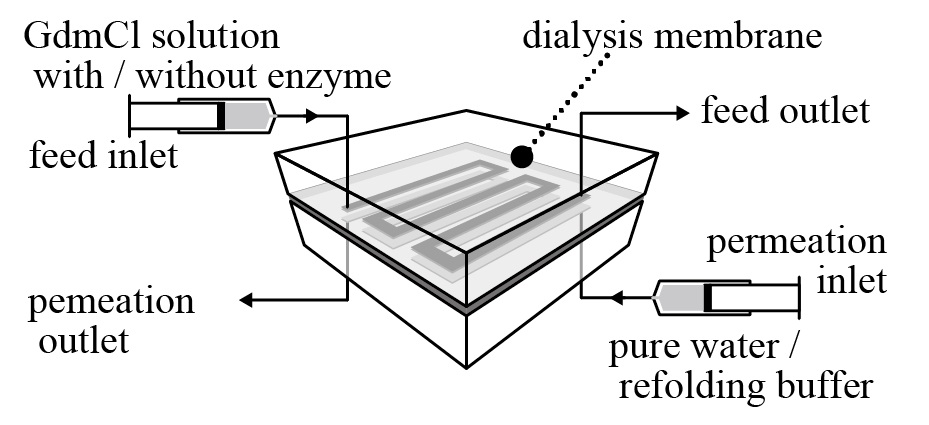
This paper offers how to realize quick refolding of high concentration protein via microchannel flow membrane dialysis. E. coli. are suitable host cells for mass production of proteins due to their fast proliferation and translation rate. On the other hand, proteins produced by E. coli. are often expressed as insoluble inclusion body, which have no activity. In that case, the “refolding” process to recover the original protein structure and activity is required. A quick refolding of high-concentration protein is important issue for industrial process but generally quite difficult. Dilution method can quickly refold protein but the concentration becomes low, while dialysis method can refold high concentration protein but requires long time. Considering the rate determining step of the dialysis is the slow permeation of denaturant through dialysis membrane, here, we propose the design to realize both of quick and high-concentration protein refolding by using microchannel flow dialysis.
Dialysis membrane was sandwiched with two microchannels; feed channel and permeation channel. Carbonic Anhydrase (CA) (as model enzyme) denatured by denaturant (guanidinium chloride (GdmCl)) was fed into feed channel. On the other hand, refolding buffer was fed into permeation channel. The denaturant was quickly removed from feed channel to permeation channel through dialysis membrane due to high specific membrane surface area in microchannel flow. As a result, CA can be refolded in only twenty minutes, which are much shorter than the time for conventional dialysis method, and just a bit longer than the time required for refolding of CA. Furthermore, the microchannel flow process realizes almost 100% protein recovery and active recovery for CA with concentration as high as conventional dialysis method within the quick process. The demerit of the microchannel flow is low flow rate but it can be easily solved by numbering up and increasing flow rate with longer channel flow.
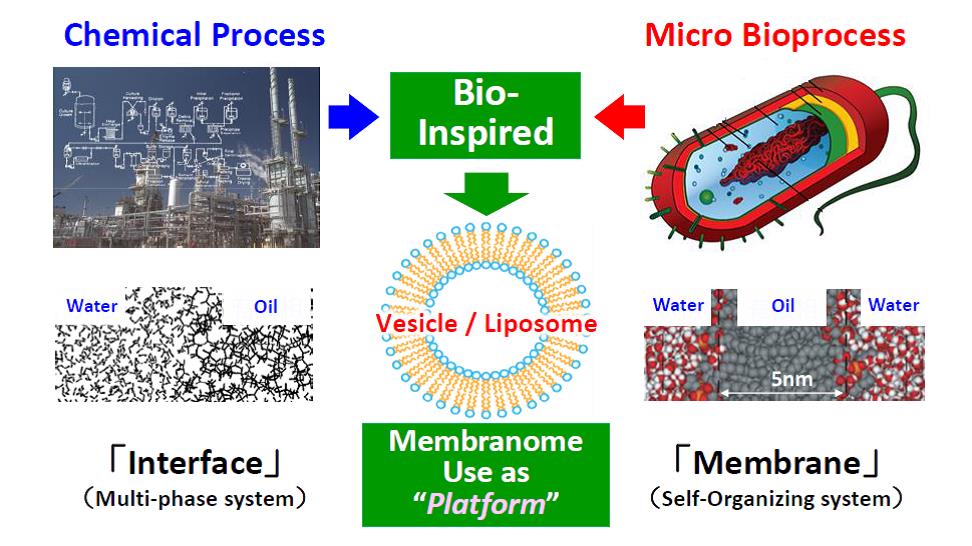
A “Biomembrane” is a highly-organized self-assembly of biomolecules (i.e. lipid, protein etc.) and a key interface for the survival of biological cell. The “Membranome” can be defined as the properties of vesicle (or liposome), which arise from the bilayer molecular assembly of amphiphiles, focusing on “emergent properties” which are not present in the individual components, and is gradually recognized as an important research methodology to investigate the potential functions of vesicles (or liposome) and to apply them for the bioprocess design. “Self-Organizing System”, such as liposome or vesicle, possesses several benefits in the recognition of (bio)molecules, where it can recognize them with (i) electrostatic, (ii) hydrophobic interaction, and (iii) stabilization effect of hydrogen bonds at its surface. A key of next chemical engineering is the use of “Self-Organizing System”, where “enthalpy-driven” nature of chemical process would be converted to “entropy-driven” one. We call this strategy as “Bio-Inspired Chemical Engineering”. In this study, the basic and applied aspect of the self-organizing system were reviewed, especially focusing on chiral separation and chiral conversion process.
Reference:
P. Walde et al., Chem. Commun., 50, 10177-10197 (2014). T. Ishigami et al., ACS Appl. Mater. Interf., 7, 21065-21072 (2015). T. Ishigami et al., Langmuir, 32, 6011-6019 (2016). M. Hirose et al., Langmuir, 31, 12968-12974 (2015). F. Iwasaki et al., ACS Omega, 2, 91-97 (2017). F. Iwasaki et al., ACS Omega, 2, 1447-1453 (2017).
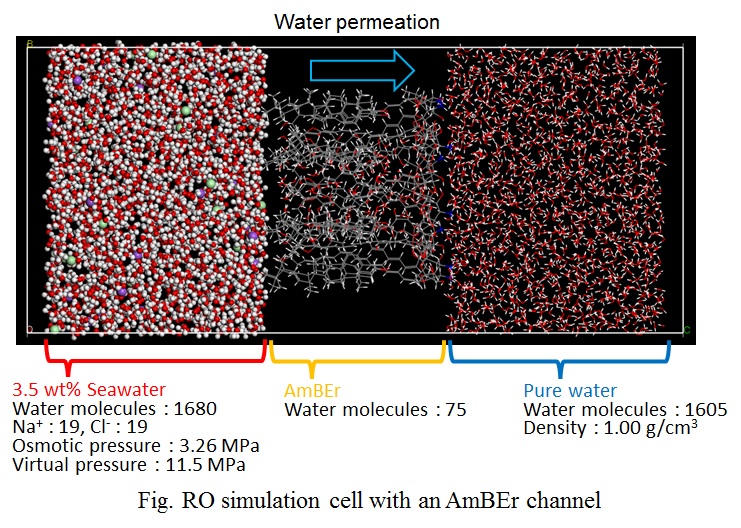
Polyamide and cellulose triacetate are known to be materials for reverse osmosis (RO) membranes, which are used in desalination of seawater. In order to deal with increasing water demands all over the world, a development of higher performance RO membrane and membrane processes are required. A drastic change in RO membrane materials would lead to one of the solutions to improve RO membrane performance. Amphotericin B-Ergosterol (AmBEr) is a biomimetic channel, and water and specific ions can be selectively transported through the channel [1]. RO membranes in which AmBEr channels can be embedded suitably are expected to be utilized high performance RO membranes. In order to design high water permeable channels, it is indispensable to understand microscopic channel structures and water transport mechanisms in a molecular scale.
In this study, a quasi-non-equilibrium MD simulation technique with applied (RO mode) or osmotic (forward osmosis, FO mode) pressure difference of several MPa was conducted to estimate water permeability through an AmBEr channel (Fig.). We had successfully carried out direct simulation of forward osmosis (FO) water permeation in artificial water channels [2]. In RO simulation, the virtual pressure was applied by increasing density of seawater side in RO simulations. Simulated FO and RO water permeability for an AmBEr channel were equal to or greater than the water permeability in Aquaporin [3] and carbon nanotube [4] water channels. No permeation of Na+ and Cl- ions were observed during the simulation time of several nanoseconds. The results suggested the excellent performance of AmBEr channel as an artificial biomimetic membrane material.
[1] H. Wu et al., J. Memb. Sci., 545, 229 (2018), [2] H. Wu et al., Desalination, 424, 85 (2017), [3] M. Kumar et al., Proc. Natl. Acad. Sci., 104, 20719 (2007), [4] B. Corry, J. Phys. Chem. B, 112, 1427 (2008)
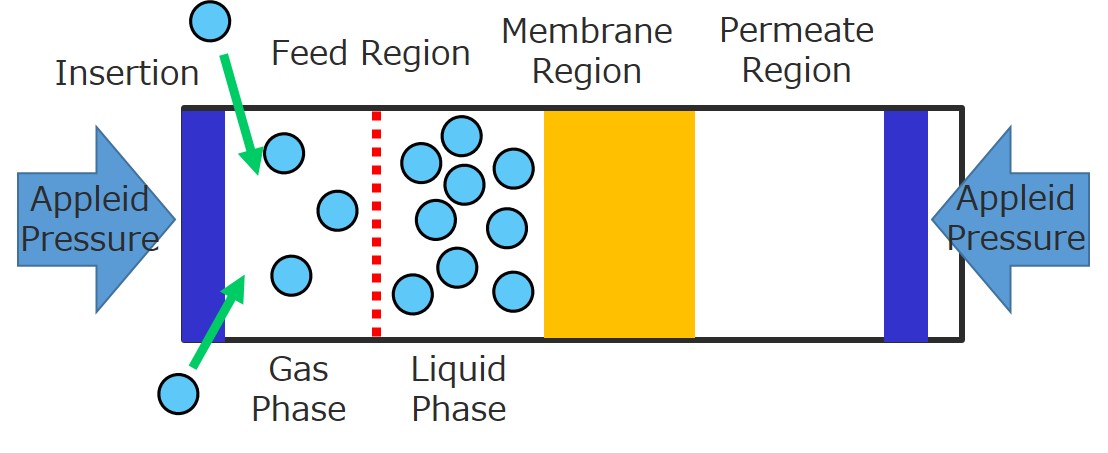
Nanofiltration has been applied to various separation systems, in particular, for the separation of aqueous or organic solvents. For a development of more efficient membrane process, better understanding of separation mechanism of solvent including multicomponent species is of fundamental. Molecular modeling becomes a powerful tool for prediction of membrane performance and design of membrane materials for particular separation systems. Our group has reported a novel simulation scheme of molecular dynamics for the permeation of feed solution through nano porous membranes. Molecular dynamics of multi component species in the feed, however, is still difficult to be simulated because a feed concentration of multicomponent such as ions cannot keep at a constant in our simulation techniques as well as other conventional non-equilibrium molecular dynamics scheme. In this paper, to model multicomponent solutions through nanofiltration or reverse osmosis membranes, we will present a novel molecular dynamics technique that can control the feed concentration including multicomponent species at constant. This simulation technique is completely new and first methodology that could model multicomponent solution systems in membrane separation from atomistic level, as long as our knowledge. We will report that this simulation technique works well and can produce the permeation of multicomponent feed solution. The flux and ions selectivity were calculated and compared to the theoretical values to examine the validity of our proposed scheme.
We have synthesized 80-cm-long mordenite membranes on mullite supports by secondary growth method. Mordenite has a moderate Si/Al ratio of 3–10 and regular pore size of 0.65 × 0.7 nm. These advantages allow mordenite membranes to be a potential candidate for separating water from acetic acid solutions with excellent acidic-resistant property and hydrophilicity. The enhancement of flux as well as high reproducibility is critical for industrial application of mordenite membranes. To improve the flux of membrane, we rapidly prepare mordenite membranes on macroporous mullite supports in a fluoride-containing precursor gel. The molar composition of synthesis solution is 1SiO2: 0.08Al2O3: 0.25Na2O: 0.2NaF: 40H2O. The hydrothermal treatment is carried out at 170 °C for 5 h. After synthesis, the as-synthesized membranes are characterized by XRD, SEM and pervaporation (PV) test. The surface SEM image of the membrane prepared under optimal conditions shows compact and highly intergrown zeolite layers composed with ellipsoidal polycrystalline grains. The membrane thickness is approximately 8 μm. High-flux mordenite membranes exhibit a long-term acid stability for a 90 wt% HAc/H2O mixture at 75 °C, the flux and separation factor ultimately keep stable at approximately 1.03 kg m-2 h-1 and 4500 for 10 d. Furthermore, mordenite membranes are successfully scaled-up from 10 cm to an industrial scale of 80 cm with transverse crystallization. These high performance 80-cm-long mordenite membranes with good roeprducibility show a promising industrial application for dehydration of water-acetic acid mixtures.