In generally, permeation phenomenon in microporous membrane can be divided in three steps, adsorption, diffusion, and desorption. Though investigation of these factors in membrane is quite important to understand permeation mechanism, these factors are hardly evaluated in direct.
We previously reported non-destructive adsorption measurement for zeolite membrane.1 In this study, we estimated diffusion property of hydrocarbon in silicalite-1 membrane from gas permeation measurement.
Silicalite-1, pure silica zeolite with MFI-topology, membrane was synthesized by a seed-assisted crystallization method on an outer surface of porous tubular a-alumina support (Noritake, i.d. = 7 mm, o.d. = 10 mm, average pore size = 150 nm).
Diffusion and desorption behavior was evaluated as follows. At first, permeation flux of helium through silicalite-1 membrane was detected. After that hydrocarbon vapor were fed to a membrane with flowing helium. When micropore of zeolite membrane was saturated by adsorbed hydrocarbon, the helium flux drastically decreased. Finally, hydrocarbon feeding was stopped, and then micropore would open by desorption of hydrocarbon. Thus, we can estimate the diffusion and desorption behavior of hydrocarbon from the rate of helium flux increase by pore opening. In addition, diffusion coefficient of hydrocarbon in mocropore could be calculated by using Fick's second law.
(1) M. Seshimo, K. Matsumoto, M. Mastukata, Evaluation of micropore volume of zeolite membrane under non-destructive condition by nitrogen adsorption method, 16th International Conference of Inorganic Membrane, P2.11, July, 2016.
Acknowledgment
This work was partially supported by JST CREST (Japan Science and Technology agency, Create REvolutionary technological seeds for Science and Technology innovation program), Grant Number JPMJCR1324, Japan.
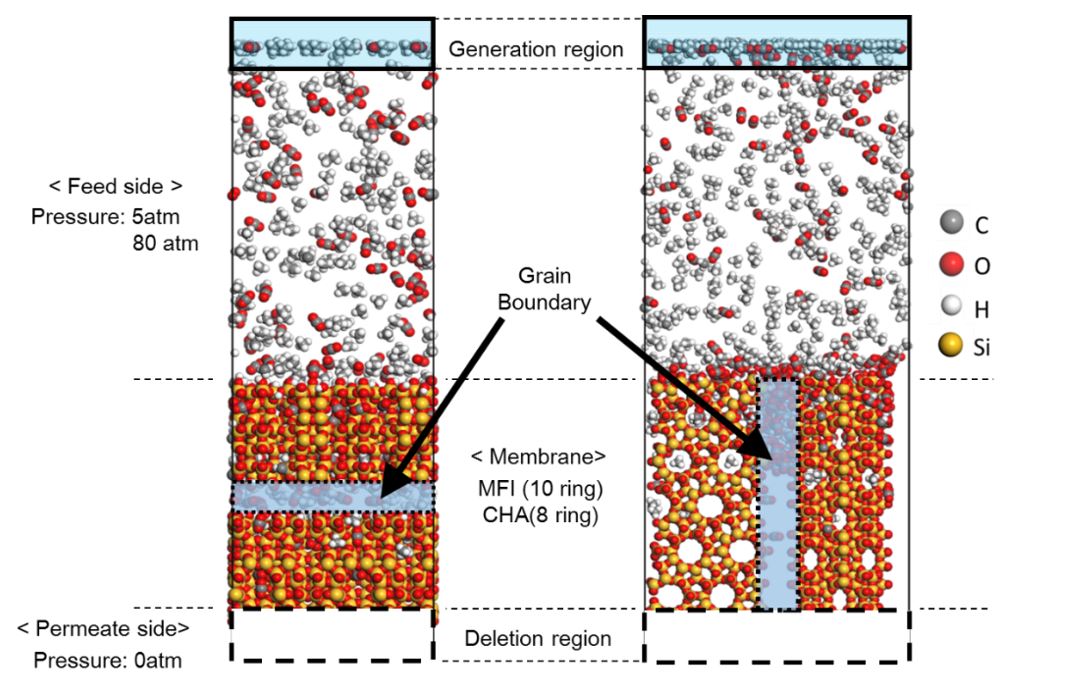
Zeolite membranes have unique properties such as molecular sieving and thermal stability. Therefore, the practical use of zeolite membranes for a purification process of natural gas has been investigated. Grain boundaries in inorganic membranes are known to degrade membrane performance, however, the detailed information of effect on selectivity still difficult to predict prior to the permeation test. Non-equilibrium molecular dynamics (NEMD) method is one of the theoretical prediction method of separation characteristics in inorganic membranes. The NEMD simulation is an atomistic-scale modeling, thus this can easily apply to the prediction of permeability and selectivity in multicomponent gas systems. The effect of trace components for membrane performance can be also investigated using NEMD.
In this study, we investigate the separation properties of CHA and MFI type zeolite membranes by NEMD for CO2/CH4 systems, and elucidate the mechanism of the effect of grain boundary on selectivity for ternary separation systems. Two different types of zeolite membranes having different pore sizes of MFI and CHA are considered to investigate the effect of pore size on selectivity. Two different models used in NEMD are shown in the figure. In the left side model in figure, grain boundary exists insides the zeolite crystal. In the right side model in figure, grain boundary runs lengthwise of zeolite crystal. In Generation region in the feed side gas molecules appear at every certain period. In Deletion region in the permeate side a permeated gas molecule is removed from the system. Our NEMD results indicates that the presence of grain boundary could increase the selectivity, which means that a control of grain boundary is a key factor to enhance the selectivity. In the conference, we will discuss the details of the effect of grain boundaries as well as pore size on this interesting phenomena regarding the selectivity.
Palladium membranes made of pure palladium, palladium-silver, palladium-copper, etc. are promising in terms of high selectivity to hydrogen. Because palladium is limited in resources and costly, various attempts have been made to reduce necessary amount of palladium preparing not only membranes supported by porous materials but also foil-shaped thin self-supported membranes. Hoverer, self-supported membranes are difficult to seal with frames of modules because welding is not easy for thin foils. To utilize self-supported membranes in practice to process large amount of hydrogen, technology to compose modules is necessary.
In this study, therefore, membrane modules for self-supported palladium membranes have been developed. A prototype module had eight sheets of palladium-silver foils 0.02 mm thick and 30 mm × 60 mm wide including sealing area. The foil-shaped membranes and spacers were alternatively accumulated and combined by thermal diffusion. The combined structure was set inside a stainless-steel container and welded between one of the spacers and the container. The module had three ports for feed, permeate and retentate gases. The module size was 34 mm × 44 mm × 64 mm excluding three ports.
Hydrogen permeation performance of the module was investigated using 1-MPa pure hydrogen as a feed gas at 623 K. As a result, 6 L(at 293 K, 1 atm)/min hydrogen was obtained from the permeate port. Durability was proved at least for 467 hours. This kind of modules are expected to enlarge the applicability of palladium membranes.
Membrane gas separation have the potential to reduce energy consumption in chemical industry. Currently polymeric membranes are widely used. However, their applications are limited because of their low selectivity. Carbon molecular sieve (CMS) membranes have higher permselectivity than commercially available polymeric membranes and lower fabrication cost than silica and zeolite membranes. The CMS membrane are expected to expand the range of application of membrane gas separation. One of problems to be considered for application is the effect of impurity. In this study, we have investigated the effect of coexisting component on gas permeation and separation through CMS membranes. Mixed gas permeation experiments have been carried out for binary mixtures of hydrogen (H2)-nitrogen(N2) and tertiary mixtures of H2, N2 and water vapor. Two CMS membranes prepared from a polyimide have been used.
Permeance to H2 at a constant partial pressure did not change with increasing N2 partial pressure for both membranes. Permeance to N2 at a constant N2 partial pressure did not changed with increasing H2 partial pressure for one membrane. However, N2 permeance increased with increasing H2 pressure for another membrane. Consequently selectivity of H2 over N2 decreased with H2 partial pressure for the latter membrane.
Coexisting water vapor decreased both H2 and N2 permeance for both membranes. However, H2/N2 selectivity decreased for one membrane and it increased for another membrane. Water vapor had a complex effect on a H2/N2 mixed gas permeation for CMS membranes.
New class of tough gel membranes containing a large amount of ionic liquids (ILs) in an inorganic/organic double-network, termed inorganic/organic double network ion gel (DN ion gel) was fabricated. The inorganic/organic double-network could be easily synthesized in the same IL pot, which allowed the preparation of freely shapeable DN ion gel, including a film shape. The DN gels showed excellent mechanical strength (more than 25 MPa of compressive fracture stress) without any leakage of ILs under compression. From tensile stress loading/unloading test, it was confirmed that the excellent mechanical strength of the DN ion gel was owing to the inorganic network which acted as a sacrificial bond to dissipate the loaded energy and the organic network which played a role of hidden length to sustain large deformation.
The gas permeation under pressurized condition was examined by using the tough ion gel membrane containing more than 80 wt% of an IL. As expected, the ion gel film was not destroyed under high trans-membrane pressure differences up to 700 kPa. Regarding the CO2 separation performance, the CO2/N2 selectivity was almost same as that of the supported ionic liquid membranes. The DN ion gel membrane with optimized network composition and 80 wt% ionic liquid sustained about 1200 barrer of CO2 permeability and 25 of CO2/N2 selectivity for more than 300 h at 50 oC under humid condition. In addition, it was confirmed that the CO2 permeability as well as CO2/N2 selectivity of the ion gel membrane containing more than 90 wt% of an IL was almost same as that of the supported ionic liquid membrane. The superior gas permeability of the ion gel membranes stem from the fact that there is almost no diffusion resistance in the ion gel owing to its low polymer content.

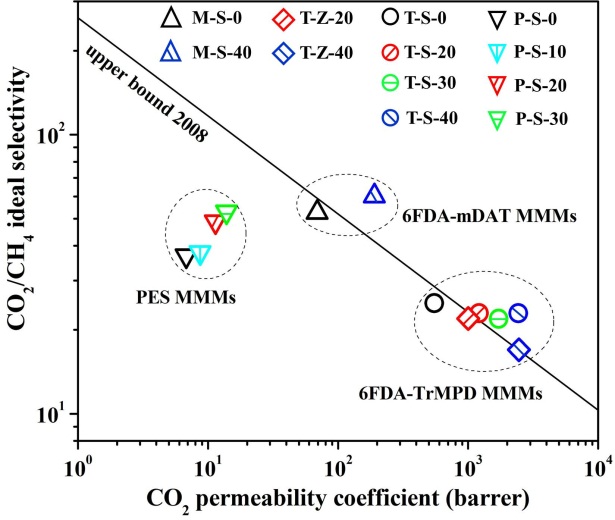
Membrane separation technology has been investigated and applied into purify natural gas, especially removing CO2 from CH4 to reduce the pipeline corrosion and increase heating value. Polymer membrane has easy processability, high reproducibility but reaches a limit in a trade-off between permeability and selectivity (Robeson upper bound line). Inorganic membrane owns excellent separation property and mechanical stability, while its high-cost membrane fabrication demands improvement. Blending inorganic particles into the polymer matrix to prepare mixed matrix membranes (MMMs) could be considered as an appropriate method due to it could combine both advantages of polymer membrane and inorganic membrane.
In this study, nanoporous SAPO-34 zeolite (~0.38 nm) and ZIF-8 particles (~0.42 nm) were severally added into polyimide (6FDA-TrMPD and 6FDA-mDAT) and polyethersulfone (PES) to prepare MMMs for CO2/CH4 separation. The filler, polymer and N-methyl pyrrolidone (solvent) were mixed to form a homogeneous solution with filler content in polymer about 0 wt.% ~ 40 wt.%. The solution was cast on a glass plate and the formed MMMs were peeled off after drying. The single gas permeation through the membranes was tested by vacuum method at 1 atm and 35 °C.
According to the figure, 6FDA-TrMPD/SAPO-34 MMMs (T-S-0~40) and 6FDA-TrMPD/ZIF-8 MMMs (T-Z-20~40) possessed the highest CO2 permeability. The 6FDA-mDAT/SAPO-34 MMMs (M-S-0~40) provided the best CO2/CH4 ideal selectivity. Addition of SAPO-34 could both increase the gas permeability and selectivity of PES/SAPO-34 MMMs (P-S-0~30) and 6FDA-mDAT MMMs. In term of 6FDA-TrMPD MMMs, all the SAPO-34 and ZIF-8 could only enhance the gas permeability at a slight loss of selectivity. Nevertheless, the separation performance of 6FDA-TrMPD MMMs containing 30%~40% SAPO-34 (T-S-30 and T-S-40) still exceeded the Robeson upper bound line (2008) of the polymer membrane, which was the same as the 6FDA-mDAT MMM with 40% SAPO-34 zeolite (M-S-40).