
Since fossil fuel combustion systems will still be used to meet the energy demand of the future, capture of CO2 represents a global environmental challenge. Simultaneously, removal of hazardous chemicals is a critical issue from environmental standpoints. Therefore, environmental regulations for industries from acceptable levels of human exposure become continuously more stringent to cope with the emission of CO2 gas as well as to protect humans from toxic chemicals. For practical applications, the developed sorbents should be structured into pellets or beads, satisfying the stability, working capacity, shape, etc.
Herein, MgO was prepared by various synthesis methods of nano-sized mesoporous MgO to optimize the platform material for MgO composite for sulfur removal and CO2 sorption. To improve the removal and decomposition efficiency of sulfur compounds, various MgO composites were developed: MgO-SiO2, MgO-Fe3O4, carbon-coated MgO, etc. The developed MgO composite showed higher performance than commercial activated carbon in sulfur removal. Then we exploit new synthesis methods for the development of porous MgO composite beads. The as-prepared spherical beads featured diameters of hundreds of micrometres, hierarchical porosity, and high surface areas. With respect to pre-combustion CO2 capture, the sorption working capacity of the salt-promoted MgO composite beads is comparable to that of powder MgO, with remarkable cyclic stability. However, at more real regeneration condition using CO2 flow, stable operation was achieved after several cycles, where the working capacity was 1.3 and 1.6 times higher than that of the corresponding powder and pressed-pellet sorbents.
Finally, in the presentation, a platform simulation model of a dual fluidized-bed system for CO2 capture and sorption enhanced reaction, which consists of a fast fluidized-bed carbonator and a bubbling fluidized-bed regenerator, will be also introduced.
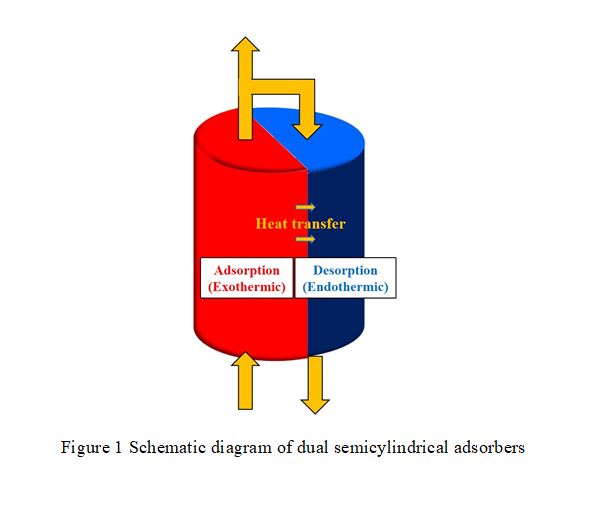
This simulation study utilizes zeolite 5A to separate oxygen from air in order to compare the performance of semicylindrical adsorber with that of traditional cylindrical adsorber for pressure swing adsorption (PSA). The semicylindrical adsorbers are designed to contact and exchange heat to each other by lateral surface area. Therefore, semicylindrical adsorber can have better heat compensation during adsorption and desorption, which increases the oxygen purity in Skarstrom cycle.
The air composition is simplified to 21% oxygen, 1% argon and 78% nitrogen. The extended Langmuir isotherm model is used to describe adsorption isotherms of gas components. Linear driving force model is used to describe the mass transfer resistance between gas and solid phase. Furthermore, the simulation program is verified with experimental data from literature. The results show the reliability of program and parameters. Then, we built a PSA process with semicylindrical adsorbers under appropriate operating conditions and compared it with a PSA process with traditional cylindrical adsorbers.
From the results of discussing the operating variables, heat compensation of semicylindrical adsorber does increase oxygen purity for oxygen separation from air, once the adsorber changed from cylindrical one into semicylindrical one. The benefit of heat compensation is more obvious for larger adsorber, higher operating and surrounding temperature, and higher feed pressure in the range of this study.
Low-grade fuel (e.g. coal and heavy oil) is one of the main sources of NOx and SOx at a time of combustion. The conventional processes for removing NOx and SOx, such as selective catalytic reduction and limestone-gypsum flue gas desulfurization, have a low NOx removal efficiency and require high cost. In our laboratory, we developed an absorption equipment having glass fiber filter, and found it attained good NOx removal performance. When the high contacting area and perfect mixing property of absorbent in this equipment are applied to simultaneous removal of NOx and SOx, there are a lot of merits, such as simplification of treatment process, reduction in operating cost, resource recycling for nitric acid and sulfuric acid, for gas treatment process. The objective of this thesis was to develop a new small model of absorption equipment for simultaneous removal of NOx and SOx, and evaluate its performance. In addition, the absorption kinetics of NOx and SOx in the equipment were analyzes. We developed new absorption equipment and indicated that NOx and SOx could be efficiently absorbed into water by using the equipment. Additionally, mixing characteristics of fluids or mass transfer characteristics in the equipment were suggested.
Up to now, many adsorbents were used for CO and CO2 separation through corporation Cu(I) to suppports. However, it is difficult to achieve high CO/CO2 separation factor, excellent CO uptake amount and large CO working capacity, simultaneously. In this work, the adsorbents were prepared by doping double metal ions consisting of divalent copper ion and various metal ion (K+, Al3+, Zn2+, Ni2+) onto MIL-100(Fe) support via impregnation method. Remarkably, the obtained adsorbent doped with copper (40 wt%) and zinc (5 wt%) showed higher CO/CO2 separation factor than the other adsorbents doped with the same concentration of copper and other metal ions (K+, Al3+ or Ni2+). The effect of zinc concentration was systematically investigated. These results proved that Cu-Zn@MIL-100(Fe) adsorbent promisses as a well adsorbent, and reveal a new route to prepare a adsorbent applied for CO/CO2 separation effectively.
Adipic acid is one of the basic materials to produce synthetic fibers like nylon-6/6. The effluent gas of adipic acid production mainly consists of nitrous oxide (N2O), oxygen, nitrogen, carbon dioxide, and water. A significant amount of N2O is emitted from the production process. Since N2O is one of the representative non-CO2 greenhouse gases, it should be recovered from the effluent gases for the mitigation of global climate change. Furthermore, the recovered N2O can be widely used in many industrial areas such as medical, automotive, and food and beverage. In particular, high purity N2O is a high value-added chemical used in chemical vapor deposition (CVD) process in semiconductor production.
Proper adsorbent selection is required for the design of adsorption separation process. In this study, to evaluate the performance of adsorbents in terms of equilibrium separation and kinetic separation, adsorption isotherms of N2O, O2 and N2 are measured on various carbon-based (activated carbon and carbon molecular sieve) and silica-based (zeolite and silica) adsorbents by a volumetric method. Adsorption amount, adsorption affinity and working capacity were compared among the adsorbents with respect to equilibrium separation. And adsorption kinetics for each adsorbent was also evaluated by applying either non-isothermal model or isothermal dual resistance model to experimental uptake curves. The physical properties and adsorption characteristics of each adsorbent-adsorbate system were used to each model. The validity of the equilibrium and kinetic results was confirmed by comparing the experimental breakthrough curves with the dynamic simulation results using the obtained values.
ACKNOWLEDGEMENT This research was supported by the Design Technology Development of Hydrogen Production Systems (100,300 Nm3/h) for Hydrogen Refueling Station (RD2017-0421) funded by KOGAS research institute.