
Metal-organic frameworks (MOFs) offer many interesting opportunities in adsorption technology, with unprecedented capacities and chemical and structural tunabilities. A great deal of effort has been made to develop a method of synthesizing MOFs, including solvothermal, microwave-assisted, sonochemical, mechanochemical, and electrochemical techniques. Among the many MOFs, zeolitic imidazolate framework (ZIF) is undoubtedly the most extensively studied because of its facile synthesis coupled with its exceptional chemical and thermal stabilities. Controlling the crystal size and shape is crucial for regulating the structural flexibilities and mass transport properties.
Herein, we describe highly versatile and effective strategies to produce polycrystalline ZIF particles and membranes. The spray-drying method has been applied to fabricate the polycrystalline ZIF particles. The polycrystals with grain boundary structure enhanced both adsorption capacity and uptake rate. The support-surface activation has been applied to fabricate the polycrystalline ZIF membranes without the use of seed crystals. The flexibility tuning related with grain size could control the membrane separation performances.
The proposed synthesis strategies open new opportunities for creation and morphology-control of new MOFs.
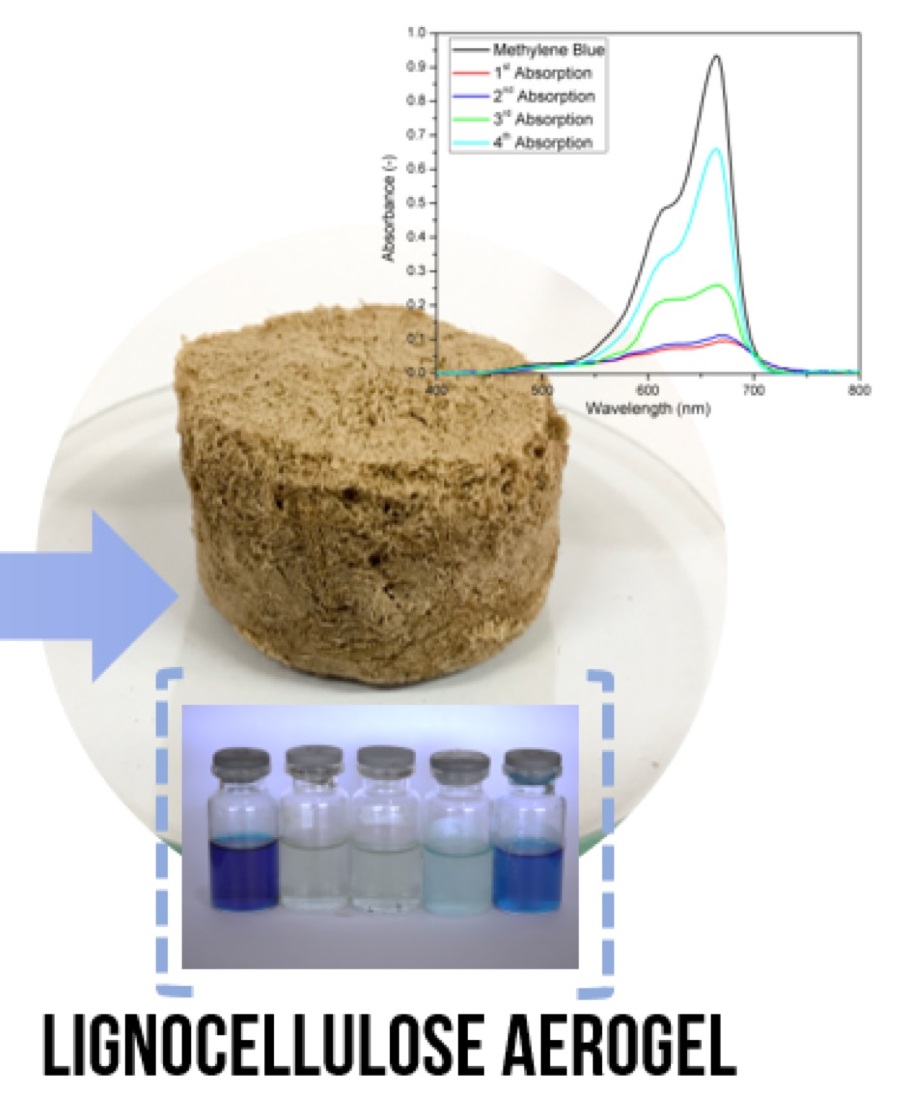
Multi-functional sorbent derived from biodegradable lignocellulose aerogels have been successfully prepared from coir fibers with sulfur–free method and NaOH-urea system. Sulfur was avoided during pretreatment because of its environmentally harmful and disadvantageous for sorption applications. Interestingly, these pretreatments had strong effect on the physical properties of the aerogels produced. Excellent physical properties of lignocellulose aerogel can be obtained when the Kappa number, i.e., the lignin content, in the pulp is lower than 14.8. The aerogel has a macroporous structure, ultralight density, high porosity, good durability, and thermal stability. The lignocellulose aerogels were capable of absorbing approximately 22 times their dry weight of water and approximately 18 times their dry weight of oil. For dye adsorption, the cellulose aerogel could absorb methylene blue molecules up to 62 g/g aerogel, hundred times higher than those of synthesized from natural-origin adsorbents. Therefore, the prepared lignocellulose aerogels have great potential for various applications including liquid absorbents and dye adsorbents.
The separation of protein, which influences on the purity and the production cost of downstream processing, plays a critical role in the protein production process. The affinity separation method using a ligand is often used for separation of protein. In this study, a functionalized membrane was prepared by immobilizing cibacron blue (CB), a dye with an affinity for specific proteins, on a polyvinyl alcohol (PVA) nanofiber nonwoven membrane produced by an electrospinning method. To evaluate its separation performance, the adsorption test of bovine serum albumin (BSA), a kind of proteins, was conducted using it. The CB molecules were immobilized by covalent bonding of hydroxyl group of PVA and chlorinated triazine ring of CB under alkaline condition. The adsorption test of CB-enhanced affinity PVA nanofiber membrane was conducted by soaking it into the BSA solution as an adsorbate. After immersing the PVA nanofiber membrane in 15 ml of the BSA solution and shaking for 4 hours, the adsorption amount of BSA was obtained from the reduction in the concentration. Furthermore, the dependence of pH was also examined by testing the BSA adsorption amount under various pH conditions. As a result, after enhancing affinity by CB, the BSA adsorption amount of the PVA nanofiber membrane indicates 2.28~3.35 times increase due to functionalization. The adsorption amount of BSA became a maximum at pH 5.0, which is the isoelectric point of BSA. In conclusion, the BSA adsorption performance of the PVA nanofiber membrane could be greatly improved by the modification with CB. Moreover, the adsorption characteristics were heavily dependent on the solution environment.
The main aim of this study is to investigate the removal of phenolic compounds as pollutants in palm oil mill effluent (POME), the wastewater from palm oil industry, by palm kernel shell activated carbon (PKSAC). In the first, palm kernel shell (PKS), one of the wastes from the industry, obtained from Thailand was characterized by proximate and ultimate analyses, and FTIR one. Secondly, PKS was thermally treated to produce PKSAC by chemical activation with orthophosphoric acid. The yield of PKSAC ranged from 0.36 up to 0.72 and decreased with increasing temperature or time in the thermal treatment. The specific surface area of PKSAC (SPKSAC) was 400×103-800×103 m2 kg-PKSAC–1 and the highest at 673 K of thermal treatment temperature. The longer treatment time reduced SPKSAC. The types of functional groups on PKSAC surface were less than those on PKS surface and new functional groups of phosphorus species formed. Then, batch equilibrium adsorption of the model POME was conducted to remove phenolic compounds in POME using the PKSAC. The PKSAC could adsorbed phenol and m-cresol in the model POME and remove them from the solutions successfully. The adsorption isotherms of both phenolic compounds followed the Langmuir model. The saturated adsorbed amount of m-cresol was higher than that of phenol. This may result from higher hydrophobicity, i.e., lower solubility in water of m-cresol than phenol. The saturated adsorbed amounts of phenolic compounds increased with SPKSAC. The effect of the functional group types on PKSAC surface on the phenolic compound adsorption was unclear. Finally, the treatment process of POME with the PKSAC was discussed with the above results based on simple relationship of material balance and adsorption equilibrium. The treatment process would be expected as a promising treatment method since the PKSAC gave high saturated adsorbed amount with high yield production.
Biosorption is an eco-friendly, low-cost method and suitable for heavy metal adsorption among conventional methods of metal ion removal. Au(III) and Cu(II) adsorption from aqueous solution were carried out using low cost, biosorbent materials of sheep wool and chemically treated sheep wool. Sheep wool is a keratin-based natural fibrous material and contains many active functional groups. NaOH, Na2S, NaHSO3, and NaBH4 were used as chemical reagents for the treatment of the wool. The morphological and chemical characterization of the chemically treated wool and wool samples were performed by the Scanning Electron Microscopy and Fourier Transform Infrared Spectroscopy. The fibrous wool was transformed into a film like structures after the chemical treatment of Na2S. The functional group of amine was determined as being the main active site for the adsorption of Au(III) by FTIR analysis. The sheep wool and the chemically treated sheep wool samples adsorbed Au(III) from Au-Cu binary aqueous solution and the presence of copper ion had no effect on the Au(III) adsorption. Au(III) adsorbed substantially at low pH range. The experimental data fitted well with the model of pseudo-second-order kinetic and the adsorption amount of Au(III) adsorption increased with time and reached a plateau after 12 h. The rate determining step of Au adsorption would be defined as chemisorption due to the formation of a monolayer on the wool surface. Sheep wool is a low cost, eco-friendly material, and has a high capacity of adsorption, and could be used as a biosorbent for precious and heavy metals by modification of appropriate chemical treatment.