
Glucose and xylose, which are the main components obtained by hydrolyzing biomass such as waste of agricultural products and plants, could be used in ethanol fermentation. However, the uptake kinetics of each the sugar may drastically change when glucose/xylose mixture is given to yeast as a carbon source for the ethanol fermentation. The objective of this study is to apply the developed quantification method to the determination of metabolites contents in ethanol fermentation media based on infrared spectroscopic information.
Fermentation was conducted using Pichia stipitis NBRC 10063 (NITE Biological Resource Center, Chiba) with glucose, xylose or glucose/xylose mixture as the carbon sources for 120-168 hours in yeast nitrogen based (YMB) medium. The infrared spectra of fermentation samples were collected using a Fourier transform infrared (FT-IR) spectrometer equipped with an attenuated total reflectance (ATR) accessory in the ranging from 4000 to 400 cm-1.
For the spectra of glucose and xylose in the YMB medium that represented the very similar spectral features, we extracted 14 absorption bands assigned to C-C, C-O, C-H and C-OH of the sugars and ethanol in order to characterize the spectra. Under the assumption that the spectral additivities could be satisfied, we tried to choose the best combination for the metabolite content determination in the fermentation media. Consequently, the wavenumber combination of 1036, 1050 and 1046 cm-1 was selected for the quantification, and the calculated contents of sugar and ethanol components excellently agreed with the contents measured using an HPLC method. In addition, the kinetic sugar uptake and ethanol production behaviors were accurately grasped during fermentation using glucose, xylose or glucose/xylose mixture medium based on the information estimated using the developed quantification method. These results suggested that the mid-infrared spectroscopic method would be acceptable as the simple, rapid, non-chemical and non-destructive monitoring of such fermentation processes.
Sugar fatty acid ester is one of bio-based and non-toxic surfactants. Its hydrophilic–lipophilic property can be controlled by the type of fatty acid side chain. Recently, medium-chain triglyceride (MCT), which has medium chain fatty acids (length of 6-12 carbons), has received much attention because of its preventive effect on lifestyle-related diseases. In order to use MCT in foods, its solubilization or micellization is important and hence sugar ester consisting of similar medium-chain fatty acid is required. However, such a sugar ester is not available. This is because in the industrial production method using homogeneous catalyst such as Na2CO3, reduced pressure condition is necessary to improve the product yield, but the reactant with medium-chain fatty acid also evaporates and hardly reacts under the condition.
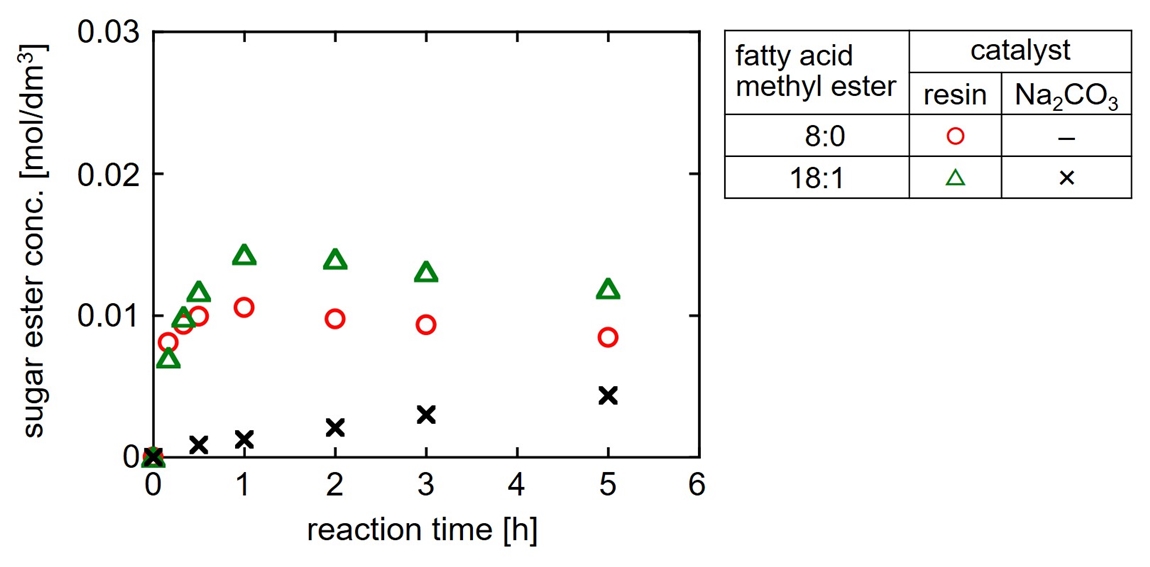
We reported the ion-exchange resin pre-adsorbed with the reactant sugar shows a high catalytic activity for sugar ester synthesis at 60 °C and atmospheric pressure1). This method has a possibility of producing sugar ester with medium-chain fatty acid which could not be synthesized conventionally. In this research, syntheses of sucrose ester with caprylic acid (carbon number: double bond number = 8:0) were discussed using microporous anion-exchange resin catalyst. In the synthesis experiments, first, sucrose was adsorbed on the resin and then methyl caprylate was added to initialize the reaction. For comparison, an experiment to produce the conventional sugar ester with long-chain fatty acid was also performed using methyl oleate (18:1). As shown in the attached figure, in the case using the resin, the conventional long-chain ester formed more rapidly than the case using the homogeneous catalyst (Na2CO3) at atmospheric pressure. The novel medium-chain ester was also formed similar to the conventional long-chain ester. Therefore, this method using the heterogeneous resin catalyst has potential to efficiently and continuously produce sugar ester with medium-chain fatty acid.
1) T. Sasayama et al., Chem. Eng. J., 334, 2231 (2018)
Synthetic biology aims to create a production system by synthetic or genetically engineered microbes. It is important to rationally design a genetic edition strategy in computer before expensive and time-consuming biological experiments. Flux balance analysis, a linear programming-based framework, have been widely used to predict the metabolic fluxes of cellular organisms in different culture conditions. Mass action models have recently been proposed to simulate large-scale cellular metabolism. However, both the models work just on the steady state; dynamic models are required to predict the cellular metabolism because synthetic microbes are often grown in a batch culture. In 2016, to simulate the dynamics of cellular metabolism, we constructed detailed kinetic models of E. coli central carbon metabolism to predict the dynamics of genetically engineered cells under aerobic condition. This model employed several major transcription factors of Cra, Crp PdhR, and IclR to consider gene expression regulation at different genetic mutants, and used detailed enzyme kinetics to take into account enzyme modification and allosteric bindings. On the other hand, useful compounds such as fermentation products are produced in anaerobic and microaerobic conditions, while cell growth are optimal under aerobic condition. Rational fermentation design of genetic microbes requests considering the mechanisms by which how dissolved oxygen affect fermentation and cell growth. Thus, we have proposed a kinetic model that predicts the dynamics of E. coli central carbon metabolism at different dissolved oxygen concentrations by considering transcription factors of ArcA/B and Fnr, respiratory chain reactions and fermentative pathways, and catabolite regulation. The model accurately predicted the metabolic characteristics and molecular mechanisms of fnr and arcA gene-knockout mutants. We used the model to design an aerobic-microaerobic dual-phase cultivation method in a pfl-knockout mutant to maximize lactate production. The model will be a platform for designing microbial cell factories to produce a variety of biochemicals.

Integration of in silico and experimental approaches is highly desired for understanding of photosynthetic organisms and creation of green microbial cell factories. We previosuly developed Genome Scale Model (GSM) of cyanobacterium Synechocystis sp. PCC6803 [Yoshikawa et al., 2011]. The developed GSM is applied to prediction of metabolic fluxes under diferent trophic conditions and to design of genetic modification for valuable compounds production. Ethanol production improvement by Synechocystis sp. PCC6803 strain [Yoshikawa et al., 2018] was designed and it was experimentally proved. Recently GSM was reconstructed with detail information of photosynthesis and understanding of electron transfer in photosynthesis system.
Metabolic flux analysis (MFA) based on quantification of 13C-labeling patterns of metabolites by mass spectroscopy (MS) is a powerful tool to quantify fluxes experimentally in a metabolic network of microorganisms. Cells are cultivated with a 13C-labeled substrate, and metabolites are extracted from the cells for use in MS analyses. Metabolic fluxes are determined using stoichiometric constraints coupled with extracellular measurements and 13C-labeling patterns of the metabolites. We developed a bioinformatics tool, OpenMebius, to determine metabolic flux from the 13C-enrichment data of isotopically labeling experiments [Kajihata et al., 2014]. Metabolic fluxes of different cultivation condition of Synechocystis sp. PCC6803 was performed and it provides new understanding of central carbon metabolism of photosynthetic organisms. Combination of the these in silico and experimental flux analysis provides us good strategies for modification of metabolic pathways to create useful cell factories of photosynthetic organisms.
Anaerobic co-digestion using biomass as a raw material has not only the advantage of energy recovery but is also a technology to reduce the environmental burden by resource circulation, prevention of global warming and creation of a recycling society.
Unutilized biomass has an important role in effective utilization of biomass. Now, there is great interest in the reuse of corn stover for renewable energy production from both output and environmental point of view. However more than half of the corn stover components are hardly decomposable organic matter. In this study, we aimed to elucidate the effect of heating as a pretreatment on biogas production of anaerobic co-digestion using corn stover from the view of a change in the physical characteristics of the raw material.
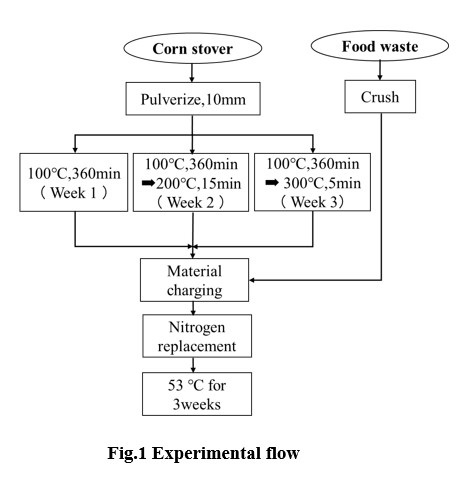
The specific heating conditions of corn stover and experimental flow are shown in the Fig1. For the raw materials, corn stover was pulverized to about 10 mm in size with a pulverizer and food waste was crushed with a food processor. About the heating pretreatment, 3 levels of samples were set and each was used as the 1st, 2nd, 3rd-week samples. The organic loading amount was set to 1.5 g-VS / L-digester / day, C / N ratio was set to 40.then the raw materials input amount was calculated. Into a 235 L volume reactor, the raw materials were charged and heated to about 53 °C.
In this study, the bulk density and open porosity of utilized corn stover were measured to explore the effectiveness of the pretreatment. The result shows that the 3rd week sample had the highest biogas production. Furthermore, the bulk density of the corn stover total solids directly affects the biogas production rate, a sample with a higher bulk density of corn stover total solids shows the lower biogas production rate.
Microalgae are an attractive and green resource with large-scale production capacity for lipid. After harvesting, microalgae always have water contents > 80 wt%. Through the traditional extraction methods, microalgae must be dried before the subsequent extraction and heated to remove the extractants after the extraction, resulting in a great increase in energy consumption; the extraction temperature is generally > 100 oC with long extraction time. Therefore, it demands an energy-saving and efficient extraction solvent.
Dimethyl ether (DME) is the simplest ether and colorless fuel gas at ambient temperature and pressure. As a commercial product, DME comes from a variety of feedstock such as fossil fuels and waste products. DME is environmentally friendly, efficient, and energy-saving as an extraction solvent. DME is partially soluble in water, rendering that drying is unnecessary for wet feedstock. The high solubility of lipid in DME ensures high efficiency. Due to a low boiling point at -24.8 oC and sufficiently high vapor pressure at room temperature, the gaseous DME after the extraction process can be removed completely and easily without heating.
Here, we conducted the DME extraction of various microalgae and firstly reported the latest development of pilot-scale DME extraction prototype for lipid extraction of wet microalgae. The wet microalgae samples with moisture contents higher than 90% grew using the wastewater at a wastewater treatment plant in South Africa. The harvesting and extraction machines also located at this plant. After harvesting, the extraction reaction of microalgae proceeded continuously at ambient temperature. Both lipid and water were extracted together and separated easily after the extraction. The yield, elemental composition, heating value, and molecular weight distribution of lipid extracted by liquefied DME were studied. The energy balance was calculated, indicating the extraction using liquefied DME as the solvent can decrease energy consumption greatly in pilot scale.