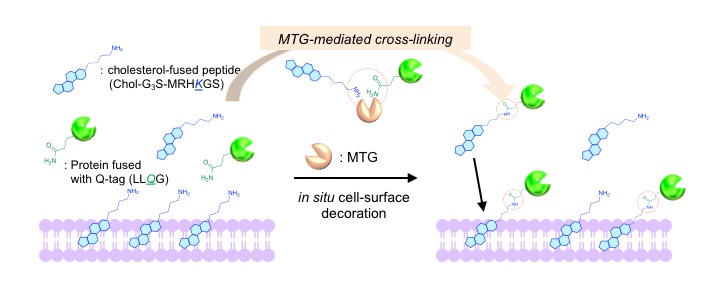
The cellular signals such as proliferation were regulated by lipid modification of proteins that are activated on a membrane-anchored state. Thus, the artificial lipid-modification of proteins is key technique in biomimetics although biocompatible modification has been challenging due to hydrophobicity of lipid derivatives. Herein, we introduce in situ decoration strategy of membrane with a protein and a synthetic amphiphilic lipid using microbial transglutaminase (MTG). MTG catalyzes the cross-linking reaction between a specific glutamine (Q) in a protein and lysine (K) in the lipid-fused peptide. In our previous report, synthesized lipid-G3S-MRHKGS lipids fully were fully recognized by MTG, yielding > 90% conversion of a protein fused with LLQG (Q-tagged protein) into a lipidated form in the presence of MTG1,2. Especially, cholesterol (Chol) containing peptide (Chol-G3S-MRHKGS) renders the conjugated protein stable anchoring ability on the cell-surface. Therefore, we applied Chol-G3S-MRHKGS for in situ cell-surface decoration with the protein: simply mixing cells to a mixture of cholesterol-G3S-MRHKGS, Q-tagged proteins and MTG. The protein decoration efficacy depended on the concentration of the subjected peptide, however, high concentration caused the cytotoxicity toward the cells, indicating the trade-off relationship between decoration efficacy and biocompatibility.
1 M. Takahara et al., ACS Appl. Bio Mater., 1, 1823-1829 (2018).
2 M. Takahara et al., Chem. Eur. J., accepted (2019).
[Introduction]
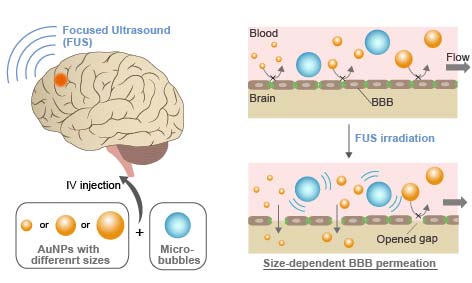
Blood-brain barrier (BBB) hamper the delivery of drug carriers into brain. Widening BBB tight junctions via focused ultrasound (FUS) can be a promising approach for enhanced nanoparticle delivery into brain. However, there is currently a poor understanding of how nanoparticles pass the opened BBB gaps. In this study, FUS-assisted BBB permeation of gold nanoparticles (AuNPs) with different size are examined in vitro and in vivo for elucidating the optimum design of nanoparticles for brain drug delivery.
[Materials and Methods]
3, 15, and 120 nm AuNPs were applied to bEnd3 cells cultured on Transwell® culture insert in vitro and ICR mice via i.v. injection in vivo under existence of Sonazoid® microbubbles, followed by FUS irradiation. TEER was measured to evaluate the barrier function of the in vitro BBB model. The amount of permeated AuNPs was measured using ICP-MS.
[Results and Discussion]
TEER measurement indicated that gaps are transiently opened for 4 h in the in vitro BBB model by FUS. The amount of permeated AuNPs in vitro was significantly increased by FUS for 3 and 15 nm NPs, while no significant difference was observed for 120 nm NPs. The permeated amount increased with decreasing NP size. FUS irradiation also significantly increased the NP delivery efficiency to brain in vivo; in the case of 0.7 MPa FUS, the efficiency increased by ca. 3-10 times. It was found that smaller particles are not necessarily better, and 15 nm NPs showed the highest efficiency. We consider that this optimum size is determined by the competition between permeation through disrupted BBB gaps and blood half-life. While smaller particles are better for the permeation through narrow gaps, they are rapidly excreted from blood by renal clearance. This hypothesis was supported by our kinetic model calculation considering NP size-dependent permeation and clearance.
Regenerative medicine using induced pluripotent stem (iPS) cells has recently attracted significant attention. To realize the therapeutic potential of iPS cells, a mass cultivation system in the undifferentiated state and efficient differentiation protocol must be established. In this study, we developed a three-dimensional method for culturing human iPS cells using hollow fibers. The cells immobilized inside the hollow fibers can proliferate and form multicellular aggregates, capable of achieving a high cell density.
We first cultured human iPS cells for under conditions that maintained their undifferentiated state. The cells proliferated and formed cylindrical multicellular aggregates that grew in the longitudinal direction of the hollow fibers. The cells maintained the proliferative activity of 7-fold expansion for 4 days in at least three serial passaging. The cells well maintained the specific pluripotent marker of SSEA-4 during passaging.
We then cultured human iPS cells for 7 days under conditions that maintained their undifferentiated state and then switched the culture conditions to allow spontaneous cell differentiation. In the 7-day undifferentiated culture, a high cell density of approximately 10-fold that of the initial seeding density was achieved. The upregulation of gene expression of three germ layers was observed in the culture of differentiated cells. Expression of the lineage-specific cell-surface marker CXCR4 (a definitive endoderm marker) was about 30% at day 5 in the differentiation culture, which was 2-fold higher than that in the traditional monolayer culture. After hollow fiber culture, we isolated the CXCR4-positive cell population using the magnetic-activated cell sorting (MACS) separator system. We were successful in obtaining a population that had potential to differentiate into a specific lineage using the efficient hollow fiber culture method.
In conclusion, the hollow fiber culture method has potential for the large-scale preparation and differentiation of human iPS cells.
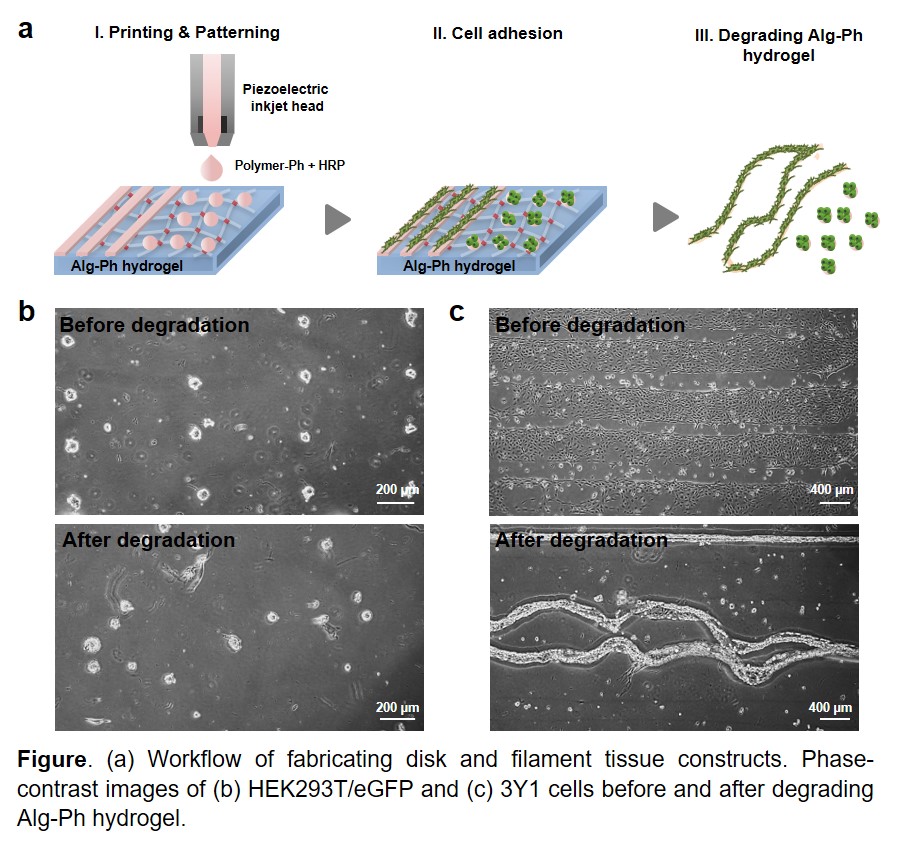
The fabrication of functional tissues has emerged as a promising technology for clinical applications, such as disease treatment and drug discovery, as an in vitro tissue/organ models. It has been revealed that the mechanical environment of tissues, determined by geometric cell patterns or material composition, play a key role in developing proper tissue function. Herein, we describe the feasibility of inkjet bioprinting with multi-ink for fabricating disk and filament-shaped tissues by patterning the cells onto substrates. Inkjet bioprinting, which is based on drop-on-demand non-contact deposition of liquids, allows the fabrication of tailor-made patterns using biocompatible materials with a simple and low-cost process. To achieve rapid hydrogelation, horseradish peroxidase (HRP)-catalyzed cross-linking was applied. HRP is a catalyst for phenol conjugation by consuming hydrogen peroxide (H2O2). A key advantage of utilizing this method includes the versatility of material choice to design various patterns. In this work, alginate (Alg-Ph), gelatin (Gela-Ph) and hyaluronic acid (HA-Ph) derivatives possessing phenolic hydroxyl groups were used. Bioink containing Gela-Ph, HA-Ph, and HRP was ejected through an inkjet nozzle head with 60 μm in orifice diameter onto Alg-Ph hydrogel sheet to form dot and line patterns that promote cell adhesion (Figure a). If the width of the gap between the dots and lines is required, a simple design modification can be done quickly with software without additional steps. Then, HEK293T/eGFP and 3Y1 cells were seeded onto the hydrogel sheets. After the optimal times of cultivation, disk and filament-shaped tissues were obtained by degrading Alginate-Ph hydrogel by adding alginate-lyase in medium (Figure b and c). We hope that this approach is promising for fabricating tissues and tailor-made cell patterning and will generate further interest in the field of tissue engineering.