
Bioprinting attracts increasing attention as an effective approach for fabricating cell-laden 3D constructs. 3D printing enables us to create physical objects from digital designs and printing precursors. The advantage of 3D printing is that it makes easy to prepare 3D constructs with different morphology, simply by modifying the digital design on computer. The aim of this study is the development of bioinks containing nanofibrillated chitosan (NC) for extrusion 3D bioprinting. Extrusion 3D printing is the most widely used printing system for developing 3D constructs. Chitosan is a well-studied polysaccharide in the field of biomaterial for medical and pharmaceutical applications because of the biodegradability, biocompatibility, and bioactivities. We evaluated the feasibility of NC as a component of bioinks for an enhancement of the shear-thinning property of the solution gellable through horseradish peroxidase (HRP)-catalyzed crosslinking of polymers possessing phenolic hydroxyl (Ph) moieties. Shear-thinning property is known to be an important property for fabricating constructs with the shape as designed in extrusion 3D bioprinting.
NC was mixed at 1 w/w% in the solutions of gelatin and hyaluronic acid derivatives possessing Ph moieties (Gelatin-Ph and HA-Ph). The addition of NC increased the viscosity at a lower shear rate but the value decreased drastically with increasing shear rate. In addition, the 3D construct obtained by extruding the ink containing NC, HA-Ph, gelatin-Ph, and HRP onto the substrate in air containing 16 ppm hydrogen peroxide was more consistent with the blueprint than that obtained from the ink free from NC. The fibroblast cells enclosed in the 3D constructs showed around 90% viability and spread in the constructs. These results demonstrate the feasibility of nanofibrillated chitosan as a component of bioinks in extrusion 3D bioprinting.
Fabrication of in vitro functional tissues which can accurately model disease condition are required for efficient drug development. Although there are a lot of neuromusclular diseases, very few drugs for them have been developed so far. In the present study, we aimed to develop in vitro functional innervated skeletal muscle tissues which can potentially model disease conditions. Previously, we developed in vitro skeletal muscle atrophy models [1] and in vitro 2D co-culture system of skeletal muscle cells and motor neurons [2]. By utilizing these systems, we developed the microdevice for 3D co-culture of skeletal muscle cells and motor neurons. The microdevices consist of two chambers; one is for skeletal muscle tissues and the other is for motor neurons. The chambers are connected each other by microtunnels which are small enough only the axons of motor neurons not cell bodies can pass through. We used the microdevices, made human skeletal muscle tissues using fibrin-based gel, and co-cultured human iPS-derived motor neurons. Glutamate selectively activate motor neurons. The constructed co-cultured tissues were actually contracted by the addition of glutamate into the chamber for motor neurons. In addition, the contraction was inhibited by the addition of tetrodotoxin which can suppress the firing of action potentials in neurons. The formation of neuromusclular junctions (NMJ) were observed by the immunofluorescence staining. These results indicated that the skeletal muscle tissues on the device were functionally connected with the motor neurons via NMJ as similar to the human body. Thus, our microdevice would be a powerful tool as an in vitro assay system for neuromusclular disease related drug development.
References:
[1] Shimizu, K., Genma, R., Goto, Y., Nagasaka, S., Honda, H., Bioengineering (Basel), 4(2):56 (2017)
[2] Yamaoka, N., Shimizu, K., Imaizumi, Y., Ito, T., Okada, Y., Honda, H., BioChip Journal, in press.
Spatiotemporal regulation of cell-material interactions has attracted a great deal of attention. Fine arrangements of cells are necessary in the field of cell engineering for applications such as regenerative therapies with artificial tissues and organ-on-a-chip techniques for drug evaluation. In addition, spatiotemporal manipulation at a single-cell level allows cells to be sorted based on their characteristics in image cytometry. Although, numerous methods have been developed for the spatio-temporal control of cell immobilization, most of these depend on cellular adhesivity for cell attachment. Thus, these methods required hours of incubation to immobilize cells and cannot be applied to cells with no adhesiveness. Rapid control of cells is essential for current “omics” analyses requiring high-throughput approaches. Non-adherent cells have also attracts growing attention in cancer immunotherapy, and detection of circulating tumor cells have significant benefits in the medical field.
Therefore, we have developed photo-cleavable materials for the quick immobilization of both non-adhesive and adherent cells, based on a poly(ethylene glycol) (PEG)-lipid conjugate (PEG-lipid) [1] and applied them to unique single-cell analysis [2,3]. Contrary to most conventional approaches that control the adsorption of serum proteins, our materials regulate the characteristics that directly binds to the cell membrane. Recently, we developed photo-switchable surfaces for the reversible immobilization of non-adhesive cells [4]. Spiropyran, a molecule that photo-isomerizes leading to a substantial difference in water solubility, was attached to PEG-lipid conjugates. This photo-switchable material enabled rapid and spatially selective in situ control of both the attachment and detachment of cells in a light-induced manner. In this presentation, this new material is mainly introduced with other recent our progresses in material-based cell manipulation technologies.
References: [1] Angew. Chem. Int. Ed., 51, 128-131 (2012); [2] Lab Chip, 31, 1933-1938 (2017); [3] Sci. Rep., 7, 14962 (2017); [4] ACS Appl. Bio Mater., 2, 33–38 (2019).
INTRODUCTION:
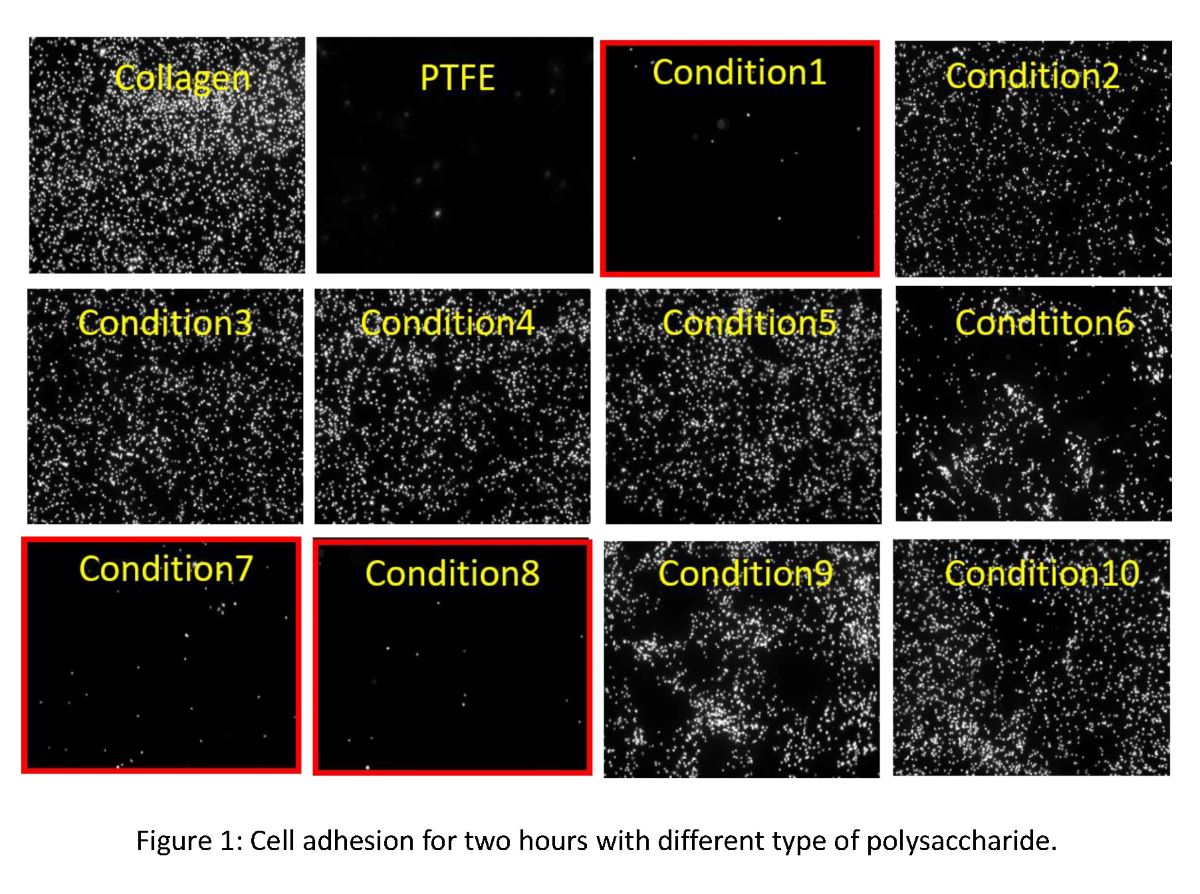
Surface modification can enhance regenerative performance of medical implants. Our group have been exploring short peptides with cell-selective adhesion/growth [1,2]. These peptides are screened from combinatorial peptide library by evaluating the dual effect on different types of cells, which enhances the adhesion/growth of cells that enhance the regeneration and inhibits the cells with negative affect on implanted area. For the application of these peptides, we have been investigating bio-compatible scaffold material for medical applications. In this work, we have developed a hybrid material of polysaccharide and collagen processed by vitrification method [3]. We here report the optimization of polysaccharide-collagen hybrid material and its peptide-modified effect for evaluating its medical usage performance.
METHODS:
Ten different types of material conditions were designed to examine the combination effect with the polysaccharide and collagen hybrid film. Their mechanical properties and cell-selective adhesion performance was investigated with endothelial cells, fibroblasts, and smooth muscle cells, which mainly consists of blood vessel and to be controlled in the regeneration of vascular tissue.
RESULTS:
We found optimized condition of polysaccharide and collagen combanied material made by vitrification. As a result, we found that our cell-selective peptides can provide cell-selective enhancement of endothelialization on newly designed polysaccharide and collagen hybrid material. The mechanical property measurement indicated that these functionalized hybrid film can show sufficient mechanical strength, and can be optimized easily by layer-forming method. Therefore, our new hybrid material can be a new candidate material to design medical implants such as sheets or vessels which can exceed the implantation of non-functionalized bare polymer material.
DISCUSSION & CONCLUSIONS: Our hybrid material combined with cell-selective material can serve as new regeneration enhancing material in medical usages.
REFERENCES
[1] Kanie et al., Materials. 2016; 9(9) 730.
[2] Kanie et al., Biotechnol Bioeng. 2012; 109:1808-16.
[3] Takezawa T, Cell Transplant. 2004;13:463-73.
Recently, treatments by transplanting tissues obtained from living cell culture have been performing as regenerative medicine. In this case, the quality of cell products varies between specimens and changes during culture. At present, quality evaluation of cultured tissue generally relies on invasive techniques. However, in the production process of cultured tissues, it is better to avoid consumption of cells for quality evaluation due to the scarcity of raw material. We aim to be able to determine the transplant timing of cultured tissue from the information obtained by non-invasive method. In this research, we considered each cellular behavior (migration, division, differentiation, etc.) as a “module”, and the strength of cell-cell adhesion and cell-substrate adhesion as energy. We constructed a cell culture simulator where all cellular behaviors are determined by energy calculation using kinetic model. In corneal epithelial cell sheet formation culture, it was observed that the fluidity of cell sheet changes during the culture. In addition, the cell sheet fluidity was different between specimens. By using simulator constructed, it was able to express the changes of cell sheet fluidity of each specimen by changing cell characteristic parameter values. As a result, it was suggested that the different cell sheet fluidity between specimens was due to the increase and decrease in cell-cell adhesion strength, and structural strength of cell sheet was high when the cell sheet fluidity was low. In addition, when the cell sheet fluidity was low, the calculated value of sum of tight junction energy in the cell sheet was maximum value. From this result, it was suggested that measurement of trans-epithelial electrical resistance, which is directly correlated with the formation of tight junction, was effective as a non-invasive and quantitative method for evaluating the strength of cell sheet structure during culture.