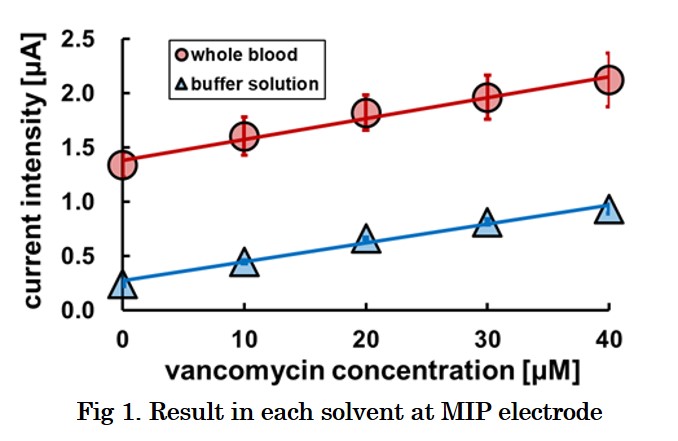
Vancomycin is the first-line antibacterial agent against methicillin-resistant Staphylococcus aureus (MRSA). Overdose of vancomycin leads to kidney damage, but underdose allows creation of the resistant bacteria. Therefore, therapeutic drug monitoring (TDM) is strongly required for chemotherapy with vancomycin. However most of hospitals outsource the analysis of the drug concentration in blood. It takes 2-3 days to obtain the data of the blood level, but there is no guarantee that the appropriate administration is done during the days. Then, we are developing a real-time vancomycin sensors using molecularly imprinted polymers (MIP). MIP is a synthetic polymer which memorizes structure of a target molecule in its matrix during its synthesis. Although their function resembles antibody but MIP can be obtained more easily and economically because their materials are same as familiar plastics. We are developing a vancomycin sensor by fixing MIP on the indium tin oxide (ITO) electrode surface. We investigated the polymerization conditions and operating conditions and attempted to develop a vancomycin sensor showing high sensitivity even in whole blood. In this study, we investigated the polymerization conditions and attempted to develop a vancomycin sensor showing high sensitivity even in whole blood. The results are shown in Fig. 1. MIP electrode indicates same sensitivity in the whole blood and the buffer solution. However, a large background current was detected in the whole blood sample, which is probably due to coexisting redox species in blood. Thus, we are trying to study the mechanism of the background-current generation and to develop the correction-method of the current.
Introduction
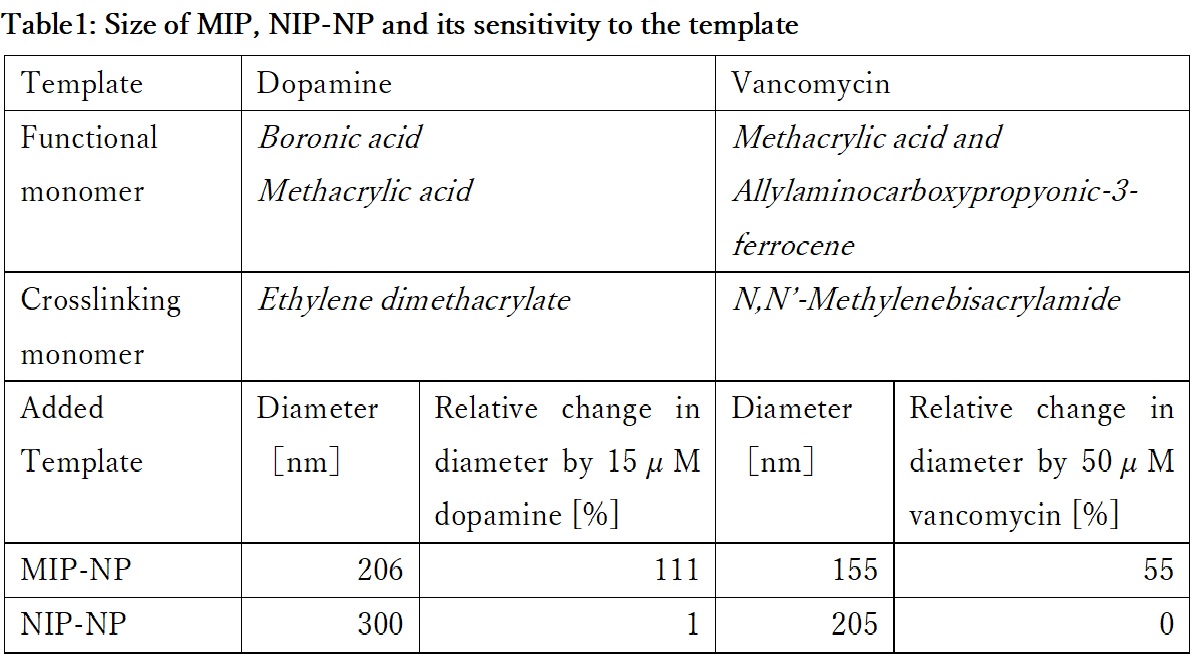
A molecularly imprinted polymer (MIP) is a polymer synthesized by molecular imprinting and characterized by binding specifically to a template substance. We are developing sensors using MIPs whose porosity is sensitive to the template specifically. In order to elucidate the mechanism of the sensor, we prepared MIP nanoparticles (MIP-NP) with the feature of increasing the particle size with respect to the template concentration. However presently, the mechanism by which MIP-NP increases the particle size is not known. Therefore, this time, MIP-NP is produced using various templates to elucidate the mechanism of MIP-NP particle size increase.
Experimental Methods
Dopamine or vancomycin were immobilized covalently on the aldehyde introduced surface of glass beads as a template. MIP was synthesized at the surface of glass beads by radical copolymerization of functional monomers and crosslinker shown in Table 1. MIP-NP was obtained by separating the MIP from glass beads surface. Non-imprinted polymer (NIP)-NP was prepared as the reference by the same operation except for omitting the template immobilization on the aldehyde introduced surface of glass beads. The particle diameter was measured by dynamic light scattering (DLS) spectroscopy.
Result and Discussion
The diameter of the particles are shown in Table 1. Each MIP-NP was smaller than respective NIP-NP. And MIP was expanded by the addition of the template, while NIP-NP was insensitive. MIP-NP possesses cavities imprinted by the template in the matrix, while the NIP does not. Those results indicate that the MIP-NP shrinks during the liberation from the template-immobilized surface and expands by rebinding with the template. Analysis of the relation between the size of MIP and the specific interaction with the template would be helpful for design of highly sensitive using MIP.
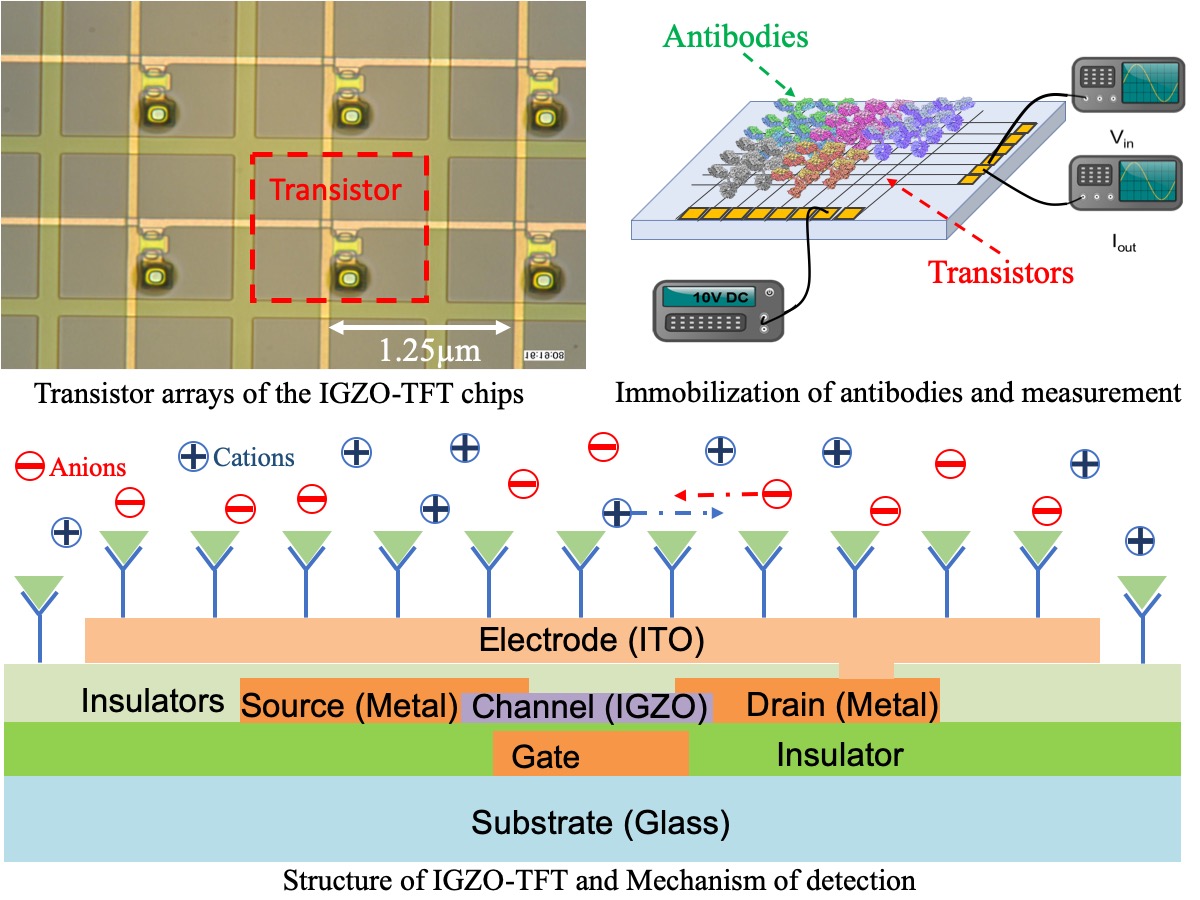
Thin film transistor (TFT) technology has been widely utilized in display, but seldomly been used in biosensing in spite of its priority in relatively high electron-mobility, economic-friendly manufacturing and lower energy consumption. We detected human serum albumin molecules by immobilizing anti-human albumin on dual-ligand modified surface of IGZO (indium gallium zinc oxide)-TFT chips with 400 transistors densely arrayed. AC power was applied on the chips and two neighboring transistors were activated. After recognition and combination, charged antibody-antigen pairs hindered movement of ions in medium between the two transistors due to electrostatic interactions, resulting in the change of permittivity of the dielectric and capacitance. By calculating output current, we could determine the impedance value, which would be reciprocally influenced by capacitance. We have detected the antibody-antigen reaction at the low level of 20 ng/ml for albumin molecules. Furthermore, we have compared 1 × PBS(Phosphate Buffer Solution), 0.1 × PBS(PBS : water = 1:9), 0.01 × PBS(PBS : water = 1:99) and 0.001 × PBS(PBS : water = 1:999) as solvents, where the results show that higher diluted PBS provides larger magnitude of signals for antibody-antigen reactions probably due to lower ion strength. The present IGZO-TFT biosensors is promising for a label-free multiple immunoassays method in the future. In next step, concentration of antigen will be decreased to determine sensitivity of the system and multiple types of antibody-antigen pairs will be immobilized and detected.
Human mesenchymal stem cells (hMSCs) are multipotent stem cells that can differentiate into mesoderm lineages such as osteocytes, adipocytes, and chondrocytes as well ectoderm (neurocytes) and endoderm lineages (hepatocytes) without generating tumors. The advantages of hMSCs used in cell therapy are their stemness, easy isolation, and no ethical concerns. These make them to serve an ideal candidate for regenerative medicine. However, the differentiated cells should be typically purified by magnetic-activated cell sorting (MACS) or fluorescence-activated cell sorting (FACS). This is laborious and expensive process. In this study, we developed a cell sorting dishes using thermoresponsive polymeric surface for isolation of targeted differentiated cells. hMSCs were differentiated into desired cell lines on the thermoresponsive surface and subsequently the temperature in culture medium was reduced below lower critical solution temperature (LCST), which enables to separate differentiated cells from non-differentiated cells. This method is an easy and economical process to be able to apply into mass production in future. Therefore, the sorting process on thermoresponsive polymeric surface should be crucial for future regenerative medicine application.
In this study, we first developed the establishment method of primary human amniotic fluid stem cells (hAFSCs) and human adipose stem cells (hADSCs) under the xeno-free condition. Subsequently, we designed the culture method of hAFSCs and hADSCs on thermoresponsive polymeric surface coated with poly(N-isopropylacrylamide), PNIPAAm, and several ECMs, such as (1) collagen I (2) fibronectin (3) synthemax II (oligo-vitronectin based substrate) (4) rVN (recombinant-vitronectin). After differentiation of hAFSCs and hADSCs into osteoblasts, we reduced the temperature in culture medium below LCST and detached the cells on the thermoresponsive surface (Fig. 1). We will investigate optimal PNIPAAm-ECM combinations for the differentiation of hAFSCs and hADSCs as well as the effect of cell sorting characteristics, which should be useful for the application in the regenerative medicine.
Microporous monoliths have been attracting increasing attention as separation materials due to their structural features. Poly(methyl methacrylate) (PMMA) is a polymer having biocompatibility and is insoluble in lower alcohols while it is soluble in the alcohols containing a small amount of water. Thus, microporous monoliths of PMMA can be made from its solutions in lower alcohols containing water via thermally induced phase separation method. In this study, we prepared composite monoliths of PMMA containing particles of hydroxyapatite (HA), which is one of the biocompatible inorganic materials that adsorbs proteins. The adsorption characteristics of proteins on the monoliths were also evaluated.
PMMA was dissolved in ethanol containing 20% water and HA powders at 70 °C. The polymer solution was then cooled for 1 h in a stainless-steel cup. After cooling, the solidified polymer was taken out and immersed in water to prepare a monolith. Bovine γ-globulin (BGG) and bovine serum albumin (BSA) in sodium phosphate buffer were adsorbed on a disk-shaped monolith in a custom-made holder and desorbed by raising the buffer concentration. The protein concentrations were determined by the bicinchoninic acid method and electrophoresis was performed to examine their adsorption characteristics.
The composite monoliths were successfully prepared by cooling the polymer solution at 0 °C. The dispersion of HA particles was improved by the pretreatment of HA powders with an aqueous solution of a nonionic surfactant Tween 80 before suspending in ethanol. The prepared monolith adsorbs much BGG and slightly BSA in 20 mM phosphate buffer (pH 6.8). The adsorbed BGG on the monolith was eluted with 400 mM phosphate buffer. The electrophoresis showed that BGG was separated from the mixture of BGG and BSA. We also examined adsorption characteristics of protein conjugates on the monolith. This study was partly supported by Kakenhi and Manufacturing Technology Association of Biologics, Japan.
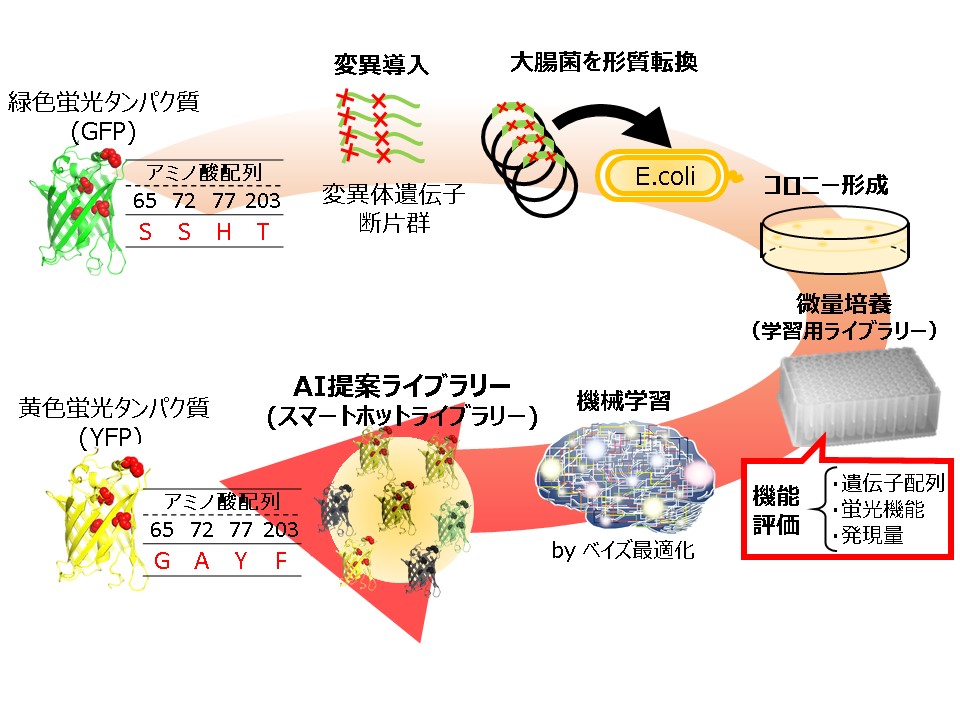
For the improvement and change of the function of protein, the amino acid residues in the protein are selected and they are mutated to appropriate amino acid residues. Error-prone PCR is one of common method to improve and change the function of proteins; however, the identified variant is not always the one with enough function: In the error-prone PCR approach, the residue positions which should be mutated might be identified, but the selected amino acid at each position is not the most appropriate residue at each position. In this study, we combined the saturation mutagenesis with error-prone PCR: positive candidate amino acids at the critical positions identified by error-prone PCR were decided by means of saturation mutagenesis, and a smart-sized synthetic library was prepared to discover the variants with most appropriate function.
Here, we applied this methodology for improve the affinity of anti-human podoplanin antibody, LPMab-16, for rat podoplanin without deactivation against human target. The single chain fragment of variable region (scFv) was prepared from the sequences of humanized LPMab-16 Fv. For improvement of the affinity for rat podoplanin, w the scFv were mutated by means of error-prone PCR positive clones were selected from the variant library by means of phage display method. In the comparison of primary structure between positive and wild-type clones, the positions which should be mutated to promote affinity were determined.
In the secondary step, saturation mutagenesis was independently conducted at each decided position and the affinity of all the variants for both human and rat podoplanins were analyzed to determine amino acid candidates. Small-sized library was generated by conducting simultaneous saturation mutagenesis with the decided amino acid candidates, and the positive clone with 27 times higher affinity for rat podoplanin was able to be identified without deactivation for the binding to human podoplanin.
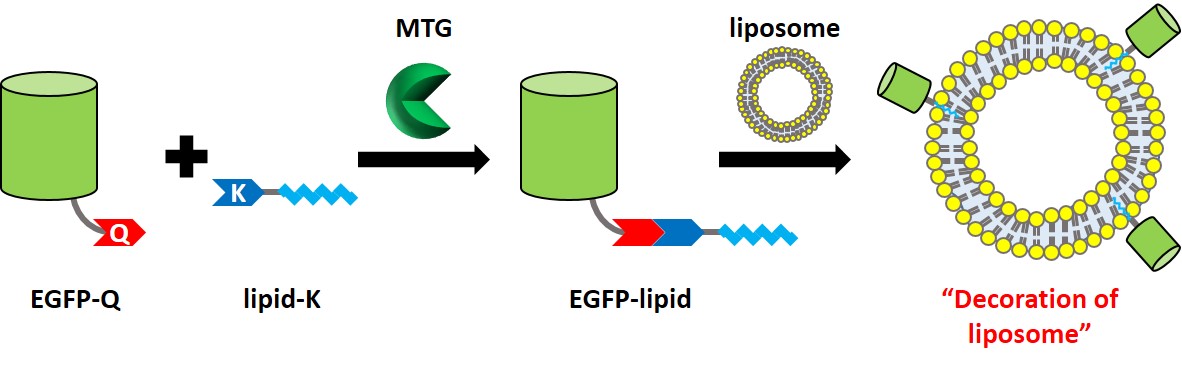
Lipid modification of proteins regulates important biological events because the attached lipid moiety activates protein function by affinity to cellular membrane. The lipidated proteins also work for anchoring on drug delivery carriers composed of a lipid bilayer such as liposome. Therefore, protein-lipid conjugate is an effective way to functionalize proteins, and the strategy has attracted attention of researchers in various fields. Herein we focused on cross-linking reaction between specific glutamine (Q) and lysine (K) residues catalyzed by microbial transglutaminase (MTG) to achieve protein-lipid conjugate. Since MTG shows strict substrate recognition and site specificity under mild conditions, it has been demonstrated an effective biocatalyst for labeling proteins with artificial lipid-fused peptide substrates [1, 2]. A recombinant enhanced green fluorescent protein (EGFP) fused with amino acid sequence containing MTG-reactive Q (LLQG) at the C-terminus (abbreviated as EGFP-Q) was expressed in E. Coli. As for K substrate, lipid-fused peptides with MTG-reactive K (lipid-G3S-MRHKGS: abbreviated as lipid-K) were synthesized by Fmoc solid phase synthesis. Prepared EGFP-Q and lipid-K were cross-linked by MTG in homogenous water-phase, and obtained EGFP-lipid after MTG reaction was purified in an established method [2]. Finally, mixing EGFP-lipid and liposomes possessing various charges led to the decoration of liposomes with EGFP-lipid, especially the combination of EGFP-lipid and cationic liposome showed the enhancement of the anchoring ability on cellular membrane.
[1] M. Takahara et al., ACS Appl. Bio Mater., 1, 1823-1829 (2018).
[2] M. Takahara et al., Chem. Eur. J., accepted (2019).
Acinetobacter sp. Tol 5 shows high adhesiveness to various abiotic surfaces from hydrophobic plastics to hydrophilic glass, and stainless steel. This adhesive property is mediated by fiber protein AtaA, which is a member of the trimeric autotransporter adhesin (TAA) family. The adhesive feature of AtaA can be conferred to other non-adhesive Gram-negative bacteria by transformation with ataA gene, and bacterial cells displaying AtaA on their surface can be quickly immobilized onto various types of supports and used as immobilized whole-cell catalysts. While it has been reported that the adhesiveness of Tol 5 through AtaA is nonspecific, material preference of Tol 5 has never been evaluated quantitatively. In this study, we compared the adhesiveness of Tol 5 among various material surfaces. Materials used in this study was polystyrene, glass, stainless steel, and anti-adhesive materials, such as polytetrafluoroethylene (PTFE), Mica, poly (oligo (ethylene glycol) methacrylate) (pOEGMA) brush, and 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer. Bartonella henselae and Yersinia enterocolitica, which adhere to abiotic surfaces through their TAAs, were used as control bacteria. The bacterial cell suspension was applied onto material surfaces. After a certain time incubation, adhered cells were stained with crystal violet and quantified by measurement of the absorbance at 590 nm. Although Tol 5 showed high adhesiveness to most of the materials, the amount of the adhered cells depended on the materials. This result suggests that Tol 5 has material preference for cell adhesion. These findings give us a clue to elucidate the mechanism of the nonspecific, high adhesiveness of AtaA.
Solid-fat microspheres (SFMSs) are micrometer-sized spherical particles consisting of solid fat having a high melting point. They can be utilized for oral delivery of lipophilic bioactive components as well as preparation of powder fats. Generally, SFMSs are produced by solidification of oil droplets in oil-in-water (O/W) emulsions containing solid fats liquefied at high temperature. However, formation of fat crystals on the surface of oil droplets during their cooling process often cause flocculation and partial coalescence, and it results in instability of SFMSs. Therefore, stabilization of SFMSs during their formulation and storage processes is needed to improve food quality and shelf life.
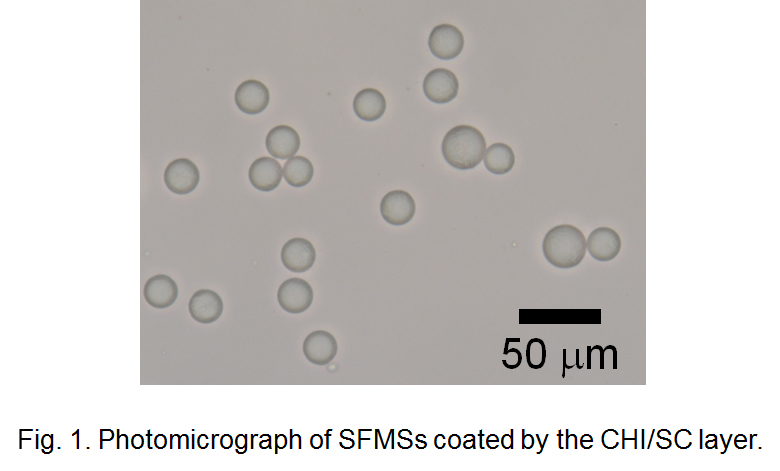
In this study, we tried to stabilize SFMSs by coating with protein and polysaccharide using the electrostatic deposition technique. Monodisperse O/W emulsions containing palm oil droplets as starting materials of SFMSs were prepared by the microchannel (MC) emulsification technique. MC emulsification is a method for preparing highly monodisperse emulsion thus it is useful for finding out the effectiveness of biopolymer coatings on the stability of emulsions and SFMSs. MC emulsification was carried out using silicon MC plates installed to MC module at 65 °C. Sodium caseinate (SC) solution was used as the continuous phase and palm oil was used as the to-be-dispersed phase. Monodisperse O/W emulsions with mean droplet diameters of a few ten micrometers and coefficient of variation lower than 5% were successfully obtained. SFMS dispersions prepared by cooling the O/W emulsion were mixed with chitosan (CHI) solution to obtain SFMSs coated by CHI/SC layer via electrostatic interaction between positively charged CHI and negatively charged SC (Fig. 1). The CHI/SC ratio affected the diameter distribution and stability of SFMSs. During long-term storage at room temperature and heating-cooling treatment process of SFMSs, formation CHI/SC layer showed remarkable stabilizing effect for SFMSs by suppressing “oiling off” and fat crystallization.
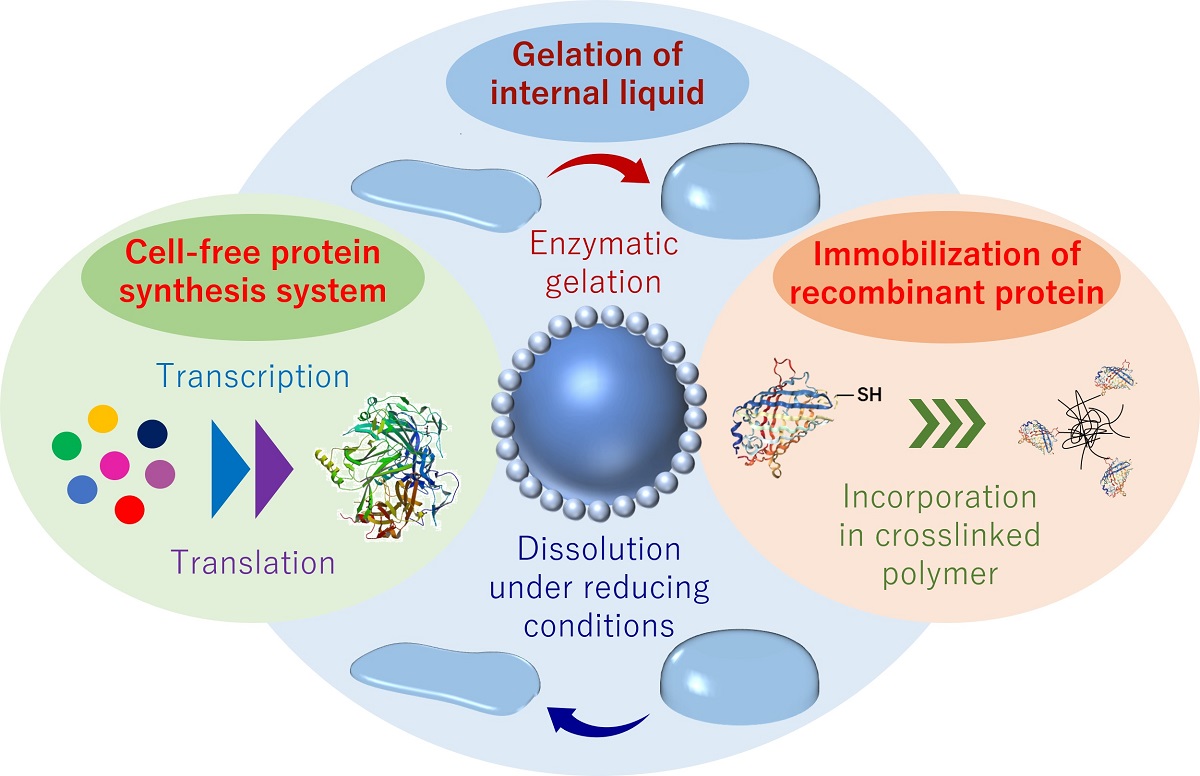
Liquid marble (LM), a self-standing microdroplet covered with hydrophobic polymeric particles, forms discrete space that can control physical and chemical exchange of substances between inner and outer environment. LM has been used as a micro-(bio)reactor because it provides easy-to-handle compartment for biological entities. In this study, we studied cell-free protein synthesis (CFPS) in LM, and the simultaneous hydrogelation of internal aqueous phase was tested to stabilize the whole system. Hereafter, we designated the LM in which the inner aqueous phase is a hydrogel state as hydrogel marble (HM). First, we constructed an LM/HM system by encapsulating the aqueous solution of CFPS components and the hydrogel precursors in LM. It was confirmed that CFPS in LM is possible under appropriate conditions (high humidity and suitable temperature) and enzymatic hydrogelation also proceeds to yield HM. As a result, capturing and immobilizing the cell-free synthesized proteins in hydrogel network was achieved, and by altering the hydrogel components and introducing cysteine residue to the target recombinant protein leaded to the efficient protein immobilization in HM [1]. We then tried to detect the gene after taking out from HM to apply the present system to directed molecular evolution. First, we prepared HMs containing the blank or target plasmid DNA encoding the gene of a target enzyme. Eventually, HMs exhibiting enzymatic activity are visually discriminated by floating HM on a substrate solution. HMs were taken out randomly, and the plasmid DNA coding target enzyme was detected only from the colored HMs. These results suggest the potential of HMs to utilize as a reactor to match genotype in LMs and phenotype in HMs. Quantitative evaluation of enzymatic activity produced by CFPS would be the next target for point-of-care testing and diagnostics.
[1] N. Kamiya, et al., Biotechnol. J., 13, 1800085 (2018).
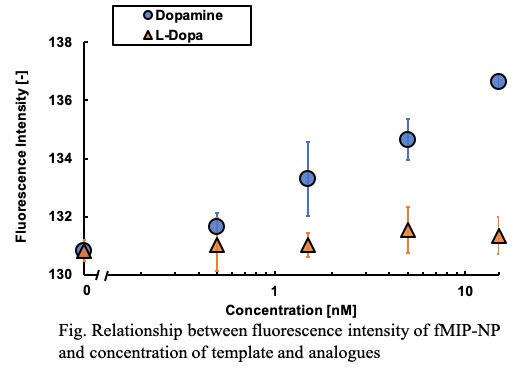
Analysis of the secretion of neurotransmitters in the nervous system is important for elucidating the mechanism of neural network. Then, development of a probe which can track a specified neurotransmitter in real time with high selectivity has been required. Molecularly imprinted polymer (MIP), which is a molecular recognition material obtained by polymerization with a template-effect of the target molecule may be applicable for the probe. In this study, nanoparticle of MIP including fluorescent group (fMIP-NP) were developed as the probe of dopamine secration. The serotonin, as the template, was immobilized on glass beads by using mixed anchor (1:1 in molar ratio) of 3-aminopropyltrimethoxysilane and 3-(2-aminoethylamino) propyltrimethoxysilane with glutaraldehyde. The template-immobilized beads were fluidized in a mixed solution of a fluorescent monomer, a template-affinity monomer, and a crosslinking monomer under UV irradiation. The colloidal fMIP-NP was collected from the surface of the beads by washing with N,N-dimethylformamide. And the dispersion medium of the colloidal fMIP-NP was replaced with 0.15 M phosphate buffer. The fluorescent intensity of the fMIP-NP was increased by addition of dopamine but was insensitive to L-3,4-dihydroxyphenylalanine (L-DOPA) as shown in Fig. The fluorescent nanoparticle prepared without the template was insensitive to both of dopamine and L-DOPA. Those results indicate that the specific interaction between the dopamine and the dopamine-imprinted cavity in the enhances the fluorescent intensity of fMIP-NP. The in vivo concentration of dopamine is said to be several nM. The relative change in the fluorescent intensity of the fMIP-NP is 4.2 % to the dopamine concentration of 15 nM which corresponds to the concentration of dopamine in vivo. Thus, the dopamine secretion in nervous system can be detected by diversion of a fluorescent voltage sensitive dye imaging system (e.g. MiCAM series in Brain Vision Inc.), which can detect relative change of 0.05% in fluorescent intensity.
Molecular evolution based on mutagenesis is widely used in protein engineering. Recent advances in genetic engineering allowed us to prepare an extremely large library, beyond the limit of organic synthesis. However, such a large library are often difficult to obtain optimal proteins due to a large sequence space and finally leads to high costs in screening experiments. The success of protein engineering crucially depends on preparing a small library with high enrichment of functional proteins. Here, we propose a novel approach that combines molecular evolution with machine learning. In this approach, we conduct two rounds of mutagenesis where an initial library of protein variants is used to train a machine learning model to guide mutagenesis for the second round library. This enables us to prepare a small library suited for screening experiments with high enrichment of functional proteins. We demonstrated a proof of concept of our approach by altering the reference green fluorescent protein so that its fluorescence is changed into yellow. A small library of GFP variants was generated by means of point saturation and site directed random mutagenesis. Sequence and functional data acquired from the variants in the library were used for training a machine learning model to create the second round library. Mix primer techniques in overlap extension polymerase chain reaction generated the second-round library which consisted of 80 variants proposed by machine learning. The library turned out to contain yellow fluorescent proteins in high number. We successfully obtained a number of proteins showing yellow fluorescence, 12 of which had longer wavelengths than the reference yellow fluorescent protein. These results show the potential of our approach as a powerful method for directed evolution of fluorescent proteins.
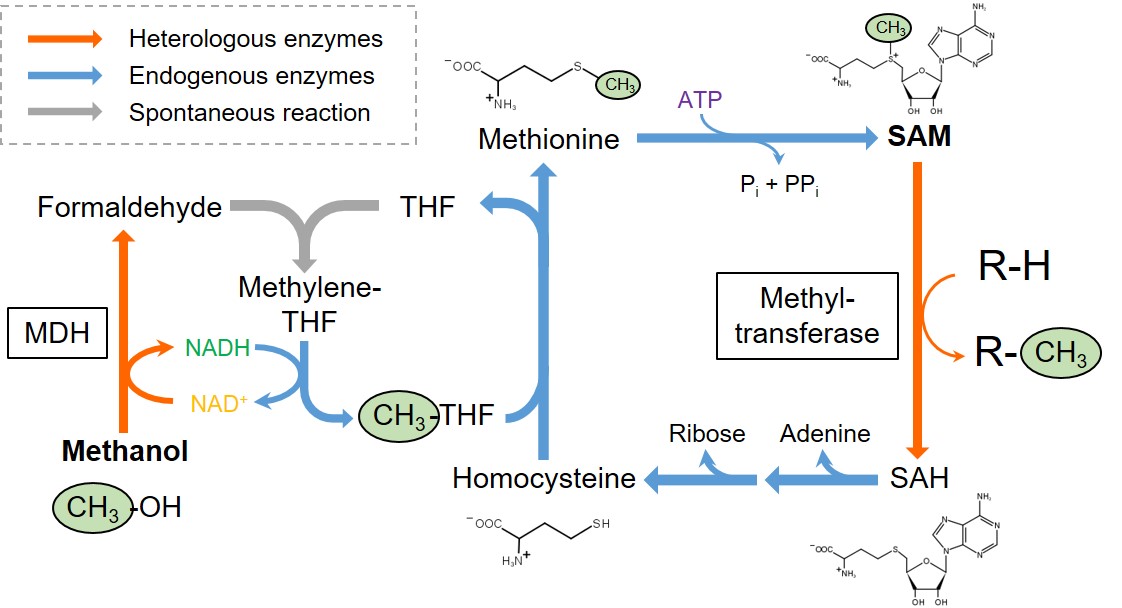
S-Adenosylmethionine (SAM) is an important methyl donor involved in the methylation of diverse biomolecules, including DNA, amino acids, and secondary metabolites. Although SAM-dependent methyltransferases can become versatile tools for the biocatalytic manufacturing of various useful compounds, SAM-dependent methylation has not yet been industrialized due to the complexity in SAM regeneration. In living cells, SAM is demethylated to S-adenosylhomocysteine (SAH) in association with the methylation of recipient substrates, and subsequently degraded to homocysteine, adenine, and ribose. Homocysteine is then re-methylated to methionine by using another methyl-donor molecule, methyl tetrahydrofolate (THF). Finally, SAM is regenerated by adenylation of methionine with ATP. Although methyl THF can be produced from serine or glycine, carbon sources taken up in the cells are mostly routed into energy production and other biosynthetic pathways, limiting the amount of carbon input which can be utilized for the production of methyl THF and following SAM regeneration.
In this study, we newly designed a SAM-regeneration pathway, in which methanol is used as a source of methyl group, and installed it into Escherichia coli. A heterologously expressed methanol dehydrogenase (MDH) catalyzes NAD+-dependent oxidation of methanol to formaldehyde, which is then spontaneously conjugated with THF to yield methylene THF. An endogenous enzyme in E. coli (methylene THF dehydrogenase) mediates NADH-dependent reduction of methylene THF to methyl THF, which serves as a methyl donor to regenerate methionine from homocysteine. We demonstrated that integration of an methyltransferase into the recombinant E. coli equipped with this pathway allowed methylation of a recipient substrate into its methylated derivative using methanol as a source of methyl group. Moreover, deregulation of SAM biosynthesys and regeneration further enhanced methylation reaction.
Krill oil contains huge amount of long chain polyunsaturated fatty acids (PUFAs) such as eicosapentanoic acid (EPA) and docosahexanoic acid (DHA). However, the easily oxidizable characteristics of DHA and EPA make a short shelf life of those compounds. To increase the oxidative stability of EPA and DHA, krill oil was encapsulated in Saccharomyces cerevisiae yeast cells. Rosemary oil (RO), an antioxidant, was added with an amount of 5 wt% of krill oil to reduce the degradation rate of EPA and DHA. The solid content of feed solution was 25 wt%, where the ratio of yeast to krill oil was 2:1. The slurry of yeast, krill oil and water were incubated at 40 °C for 4 h. Non-encapsulated oil was removed by centrifugation and washed with distilled water before spray drying to reduce the surface oil in spray-dried powder. A pilot scale spray dryer (Ohkawara L-8 type) was used to encapsulate krill oil in yeast cells. Four different powders were prepared, where RO was added in two powders and another two powders were without RO. One powder without RO and one powder with RO were washed by hexane after spray drying. Around 0.5 g powders were put in the small vial and placed the powders in drying oven at 50 °C. Thin layer chromatograph with flame ionized detector (TLC-FID) was used to analyze total oil and surface oil content of encapsulated powders. The maximum total oil and surface oil was found around 0.058 g/g-yeast powder and 0.01 g/g-yeast powder, respectively in the powder with RO and hexane washing. The content of EPA and DHA in the powder with RO had higher value than that of powder without RO after the storage of 4 weeks at 50 °C storage.
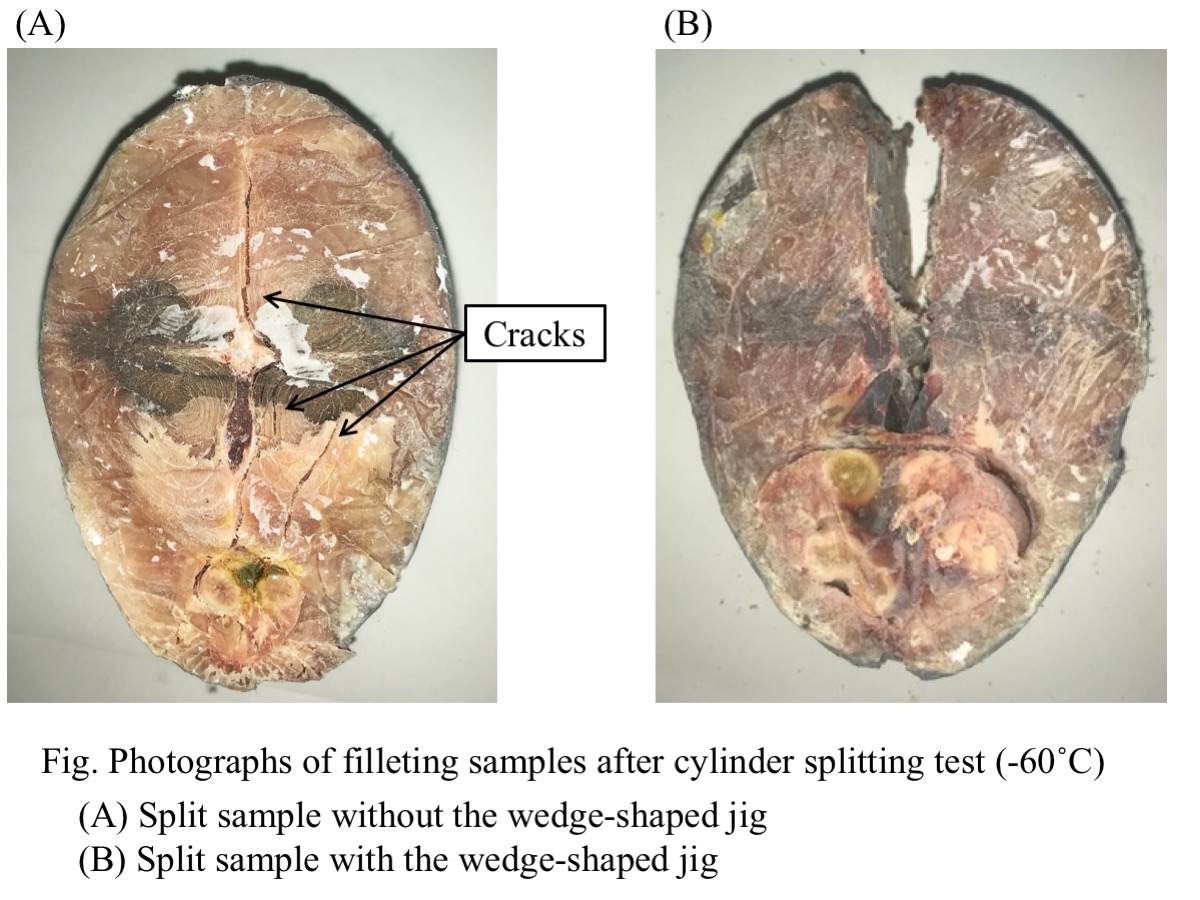
There are two methods of filleting process of large size frozen fish in the early stages of distribution : a method of cutting thawed fish with a knife and a method of cutting frozen fish with a band saw. The method of cutting thawed fish with the knife has problems in terms of hygiene and freshness. On the other hand, the method of cutting frozen fish with the band saw has problems in terms of safety and yield. We have proposed 窶彡ryo-cutting窶・as a cutting process for frozen fish without using the band saw. Samples were prepared as follows ; a thawed bonito (whole fish body) was cut into a cylindrical shape by knife and re-frozen at a temperature range from -70°C to -40°C. Notched samples was prepared by cutting a V-shaped notch on the back side of the fish sample along the dorsal fin. To try to fillet bonito, a cylinder splitting load was applied to the samples with and without the notch. A wedge-shaped jig was attached to the upper compression plate and pressed into the back side of the sample or into the notch of the samples. As a result, the sample could be split into two parts along the median plane of fish body. By using the jig, it was possible to certainly split into two parts in processing temperature range from -60°C to -40°C. Furthermore, in this temperature range, the load required for splitting could be significantly reduced by 50% and more. The reduction in stress was due to stress concentration at the notch tip. These results have shown the possibility of increasing the cutting accuracy and reducing the cutting stress, by use of the wedge-shaped jig. This study could be a new method for filleting frozen fish.
The medical application of cell engineering has attracted a lot of attention. For example, circulating tumor cells (CTCs) separated from blood offer tremendous potential for diagnosis of cancer. The analysis of CTCs is would be useful for progressions of precision medication. In order to attain those medication, it is essential to develop the cell separation technology. Centrifugation, which is based on weight and density, and affinity separation are conventional cell separation method. In addition, cell size and deformability also play a role in cell separation. A novel cell separation method based on size and deformability is worth of development, because it enables the cell separation without rare protein and marker.
In this study, we applied the metal mesh device (MMD, Figure 1.) to the cell separation. MMDs are thin metal films with a through hole structure with precisely controlled pore size. The well defined pore structure of MMD would be appropriate to separate cell based on size and deformability. The aim of this study is achieving cell separation of MMD only with the physical property of cell, and obtaining basic information.
We will report the cell separation of several cell lines with MMD. Since HL60 (floating cell) and HeLa (adhesion cell) cells were reported to have different size and deformability, the cell separation of those cells were conducted with MMD. The permeability of MMDs with different hole size was studied. We carefully observed the cells having high and low permeability, and investigated the relationship between permeability and physical properties.
Efficient oxygen supply in bioprocesses is required for aerobic microbes, which demand oxygen as the final electron acceptor in the respiratory chain reaction. In general, compressed air as babbles through a sparger is introduced in a stirring tank reactor. The bubbles sizes are ~ mm of diameters, and stirring paddles collapses and disperses the babbles, and increases oxygen transfer rate. However, the stirring blades often damage the microbial cells, and requires large input powers. To solve the trade-off problem between efficient oxygen supply and cell damages, fine babble sparger (FBS) attempted to apply the microbial cultivation. Here, influence of FBS using on a biosurfactant-, mannosyletythritol lipids (MEL), production by an absolute aerobic fungi, Pseudozyma hubeiensis were investigated. Pseudozyma hubeiensis SY62 was inoculated in 1 L of a MEL-production medium prepared in a 2-L table top bioreactor equipped an alumina porous sparger with 1.3 ホシm diameter pores (FBS sparger, Noritake Co. Ltd.). Reference cultivation were performed in the equivalent condition except without the alumina porous sparger. Cell growths were measured by a dry cell weight method and the MEL concentrations were determined by a HPLC with an evaporative light scattering detector.
Agitating at 200 rpm, cell growth at the third day with FBS sparger increased to 19.2 g/l, which was higher than that without the sparger (5.7 g/l). Although MEL production were never observed without the sparger, the production reached to 4.7 g/l for three days. However, the significant differences in cell growths between with and without FBS sparger were not observed at 525 rpm. Interestingly, MEL productions for three days with the sparger were induced to 48.9 g/L, which was 1.7 fold larger than that without the sparger. The results indicates that aeration with SPG sparger can achives an efficient oxygen supply without excess stirring.
We have developed polymer particles (g-particle) having grafted polymer chain for adhesive cell culture. To keep adhesive cells on particles surface, a graft chain having both an epoxy group and a hydrophilic carboxyl group is required. This is because the reaction between the epoxy group and the amino group of the extracellular matrix facilitates cell adhesion. The cells grown on g-particle forms three-dimensional matrix in the particles gap and three-dimensional cell matrix has many physiological functions. However, when cell suspension added to g-particle dispersion, the rate of cell growth and adhesion were not high. This was because g-particle moved freely without being dense state in the cell culture dish. In this study, to form and keep a packed state of g-particle by magnetic force, we tried to synthesize a new magnetic g-particle containing paramagnetic Fe3O4 nanoparticles. In suspension polymerization, monomer and hydrophobic modified Fe3O4 nanoparticles were polymerized and the graft polymerization of methacrylic acid and glycidyl methacrylate was carried out. Carboxyl group and epoxy group were introduced as graft polymer chains into the base polymer particles by using pre-incorporated azo groups in polymer particles as an initiator. The number average particle size of magnetic g-particles was 103 μm and they contained 5 wt% of Fe3O4 nanoparticles. They could be gathered by applying an external magnetic force. In addition, the adhesion and growth rates of cells were compared using conventional g-particles and magnetic g-particles. As a result, it was found that cell culture in the presence of magnetic g-particles shows better results.
Blood is one of the most important biological samples. This is not only an analysis target for diagnosis and research but also widely used for treatment. For example, it includes blood transfusion and blood products. Blood contains various blood cells and components that constitute plasma. Separation of these components is one of the most basic operations for its diagnosis, analysis, and utilization.
Conventionally, centrifugation is a general method of blood separation. Centrifugation is based on the specific gravity difference in principle. This is a batch method, thus, processing large amounts of blood requires large devices. In addition, it is possible to separate only target blood cells by using an appropriate centrifugation solution, however, precise separation is difficult.
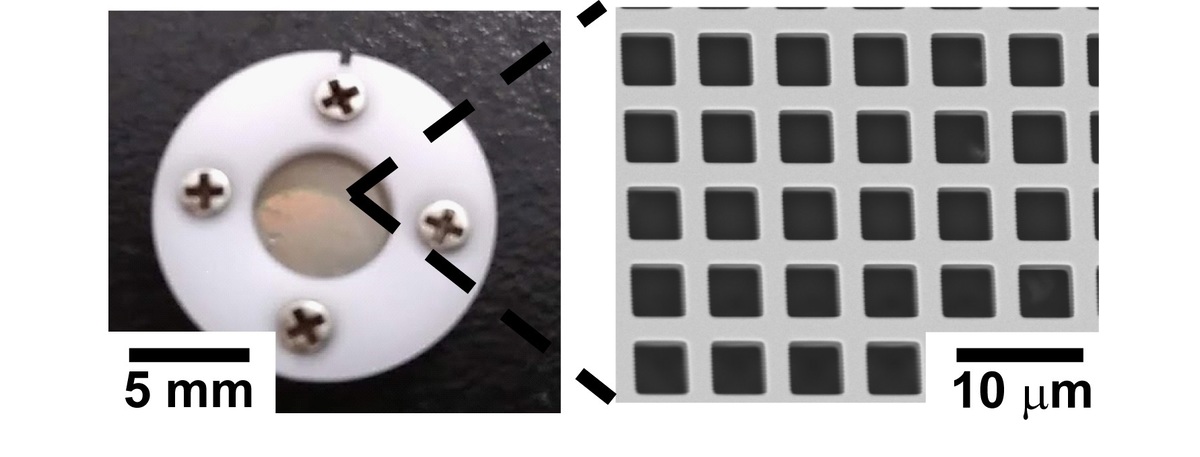
In this study, we tried that the blood cell separation using a Metal Mesh Device (MMD Fig. 1) with precisely controlled pore size and pore arrangement. The MMD has a nickel mesh structure manufactured by MEMS technology. Therefore, MMD is expected to be applied as a separation membrane, because; it has a large number of through holes accurately designed in the submicron order. It is already reported that the collection and classification of PM2.5 present in the atmosphere using MMD*.
MMD as a separation membrane has an advantageous of blood separation. That is, separation can be achieved using the simple phenomenon of passage/capture when blood is introduced. Blood cells are expected to be separated according to the size and hardness of blood cells by the mesh structure precisely designed on the submicron order. In this study, we reported the blood cell passage rate in the case of using MMD with various pore sizes and evaluated the blood cell passage control by surface PEG modification.
* H.Seto, et al., Chem. Lett., vol. 43, 4, 408-410 (2013)

Biopharmaceutical proteins such as therapeutic antibodies are usually produced by recombinant Chinese hamster ovary (CHO) cells. High producer cell lines are screened from transfected cells with random integration of target genes. Since transgene expression is susceptible to the surrounding environment of integrated genomic locus, producer cell lines should be selected from a large number of recombinant cells with heterogeneous transgene insertion. In contrast, targeted integration or site-specific integration into a characterized genomic locus enables more predictable transgene expression and less clonal variability, and hence stable production of target proteins can be expected. Genome editing technology based on programmable nucleases such as TALEN and CRISPR/Cas9 has recently emerged as a versatile tool for precise editing of target locus in the cell genome. Previously, we have demonstrated targeted knock-in of transgenes into the hypoxanthine phosphoribosyltransferase (hprt) locus of CHO cells using TALEN- and CRISPR-mediated precise integration into target chromosome (PITCh) systems [Sakuma et al., Int. J. Mol. Sci., 16, 23849-23866 (2015); Kawabe et al., J. Biosci. Bioeng., 125, 599-605 (2018)]. Here, we attempted to generate targeted knock-in CHO cells based on the homology-independent targeted integration (HITI) system. Transgene cassettes in donor vectors (pHITI/DsRed) were flanked by gRNA recognition sites to eliminate the vector backbone sequences after integration into the cell genome. To evaluate the targeted knock-in efficiency of transgene cassettes, CHO-K1 cells were co-transfected with donor vectors and respective Cas9/gRNA expression vectors. After seeding of transfectants, we counted numbers of colonies showing both drug resistance and DsRed expression. For the established clones, the targeted integration was confirmed by genomic PCR using specific primer sets for joint-regions. The transgene knock-in efficiency into the hprt locus using HITI system was 6.7-fold and 5.0-fold higher compared with those using homologous region (HR) and PITCh systems, respectively.
>Biologics are gaining importance in treatment of diseases, such as cancer, because they are safer alternatives to the conventional treatment. They are mostly produced in bacterial, yeast or mammalian cells. When there is a need for a correct protein folding and posttranslational modifications of biologics, mammalian cell lines are a go-to. Chinese hamster ovary (CHO) cell lines are the dominant mammalian cell lines in production of biologics. They do not propagate human viruses, can grow in suspension cultures, protein folding and modification is human-like and the production of the CHO cells is high.
>The demand for biologics grows. To meet the quantitative demands for the new biologics at sustainable cost, we must further increase the productivity of the CHO cells. Here, we explore the effects of cell cycle inhibitors aphidicolin and caffeine on productivity of CHO cells. We inserted an IgG1-expressing plasmid into a CHO cell lines, which were either treated with aphdicolin or caffeine during transfection, or were not treated in control samples. Aphidicolin treated cells had a 3.5-times higher productivity than control, while caffeine had no effect. While trying to identify the reason for the productivity increase in aphidicolin-treated cells, we noticed that inserted gene copy number or gene expression level remained the same between the aphidicolin treated and control cells. The inserted gene mainly in chromosome 2 in control samples, while in treated samples, the gene was found on many different chromosomes, suggesting that the location of the gene integration might influence the productivity. Because there was no change in gene expression levels between the cell lines, we hypothesize that the difference arises in the downstream steps of protein synthesis. Because the aphidicolin damages the DNA in vicinity of microRNA genes, we further hypothesize that the changes in microRNA expression might contribute to the higher productivity.
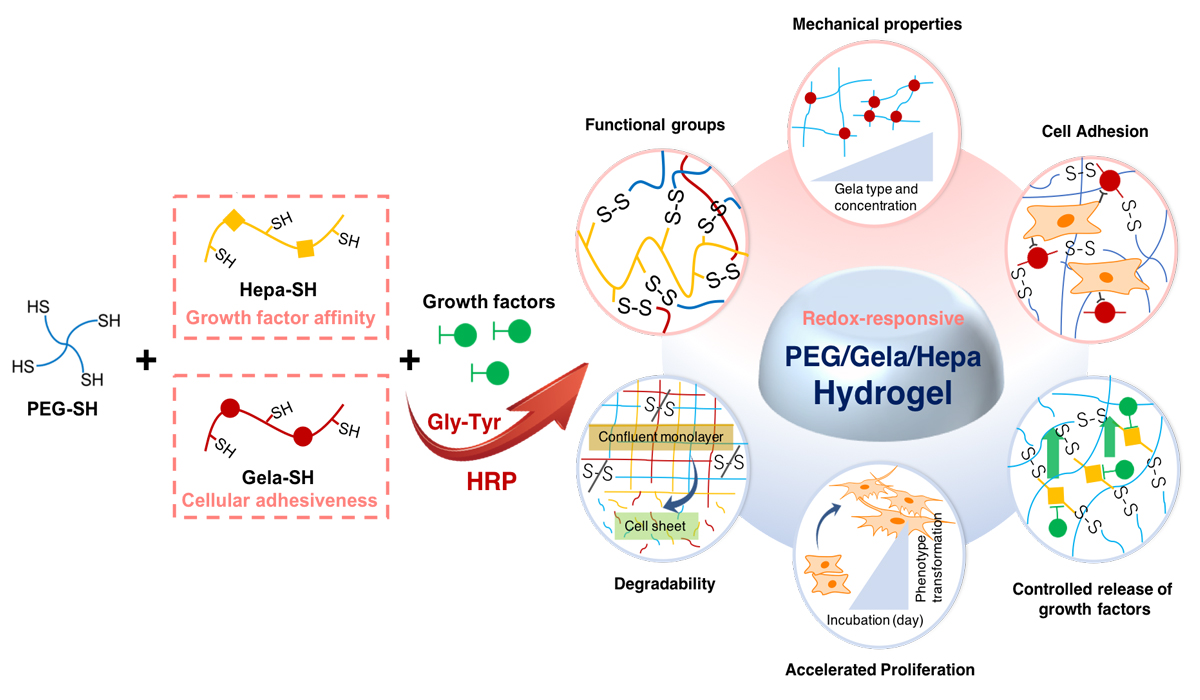
The past decade has seen a significant transition from static and passive material to dynamic, bio-based, and stimuli-responsive biomaterials in the cell-seeding technology. Recent advances towards such biologically active hydrogels have been directed to design biomaterials with a superior feature for higher-order cell culture than conventional 2D culture. One obstacle in the fabrication of biologically active hydrogels is to develop in situ crosslinking of gel precursors without impairing bioactive agents to mimic the extracellular matrix condition. Enzymatic crosslinking methods have been suggested as a potential way to introduce external functionality such as sol-gel or gel-sol transition to cell culture scaffolds. Herein, we propose to utilize the horseradish peroxidase-mediated cross-linking that can enable both redox-responsive hydrogel formation of tetra-thiolated PEG via disulfide linkage and incorporation of chemically thiolated materials in the hydrogel. Enzymatic fabrication of redox-responsive hydrogel using different types of gelatin revealed that chemically thiolated gelatin (Gela-SH) and heparin (Hepa-SH) can be co-incorporated into the polymeric network, yielding the dual-functionalized hydrogels. Hepa-SH with different degree of thiol modification was incorporated by considering its optimum binding ability with growth factors and a conflicting property such as the intrinsic anti-proliferative effect. Interestingly, the type of gelatin had a significant effect on the physical and biological aspects of hydrogels; alkaline-treated gelatin (Gela(B)-SH) exhibited superior performance over acid-treated gelatin (Gela(A)-SH) to generate the dual-functionality, cellular adhesiveness and binding ability of growth factor, originating from the two natural biopolymers. Eventually, the Gela(B)-SH/Hepa-SH dual-functionalized PEG-based hydrogel supported both cellular attachment and binding of basic fibroblast growth factor (bFGF) under cell culture conditions, which increased the proliferation (~38%) and phenotype transformation (~89%) of NIH3T3 cells cultured on the hydrogel. This hydrogel system strategy also facilitated faster fibroblast (NIH3T3) and endothelial cell (HUVECs) confluency, which makes it possible to fabricate 2D cellular sheets efficiently.
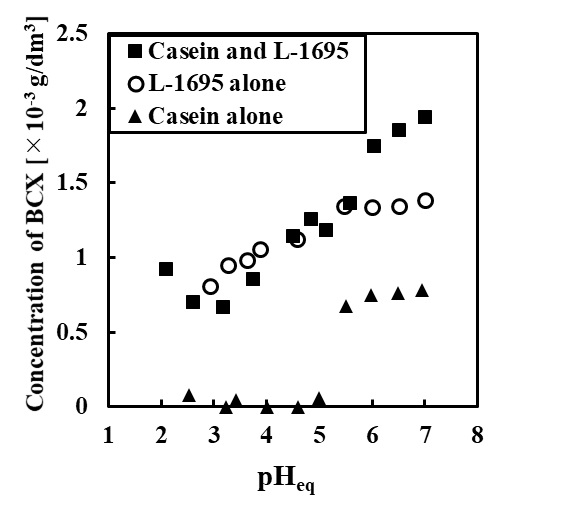
A carotenoid compound β-cryptoxanthin (BCX) is believed to have several functions for human health such as prevention of diabetes mellitus , carcinogenic inhibitory action and immunostimulatory action. However, bioavailability of BCX might be low when orally-administered, due to the extremely low solubility in aqueous media. Emulsification is one of the efficient techniques to obtain highly dispersible formulation for poorly water-soluble ingredients. In the present study, highly dispersible solid formulation containing BCX was prepared by emulsification techniques followed by lyophilization. Kumquat (Fortunella spp.) is a citrus fruit cultivated in Asian countries. BCX was extracted from dry kumquat using the mixture of pyrogallol and ethanol. An O/W emulsion was prepared using an ultrasonic homogenizer by mixing a hexane solution containing the kumquat extract, and an aqueous solution containing sodium caseinate and a sucrose fatty acid ester (L-1695) as an emulsifier. Subsequently, the resulting O/W emulsion was lyophilized to obtain a solid formulation. For the evaluation of the dispersibility, the formulation was added into an sodium phosphate-buffered solution. After filtration of the aqueous mixture using a マ・.80 ホシm membrane filter, concentration of BCX in the filtrate was determined using HPLC.
Under acidic and neutral conditions, the water-dispersibility of BCX for the solid formulation was much higher than that of kumquat extract alone. Addition of both L-1695 and sodium caseinate was effective to enhance the apparent solubility of BCX under wide pH conditions. Only in the presence sodium caseinate, the dispersibility was very small under weakly acidic condition which is close to isoelectric point for casein. Thus the solid formulation prepared by emulsification techniques was effective to enhance the dispersibility of BCX.
Hepatocytes are a promising cell source for liver tissue engineering and drug screening studies. For the success of such applications, hepatocytes have to maintain liver-specific functions at a high level. Various approaches, such as spheroid (three-dimensional cell aggregate) culture and co-culture, have been adopted to preserve hepatocyte functions in vitro. In this study, we constructed a co-cultured spheroid, which combined hepatocytes with other cells, and evaluated the effects of co-culture on the expression of hepatocyte functions.
As a culture platform for spheroid generation, we fabricated a microwell chip which contained 397 microwells (400 μm in diameter) on a poly-dimethylsiloxane substratum, and its surface was modified with 2-methacryloyloxyethyl phosphorylcholine co-polymer to create the non-adhesive area. In this study, three kinds of cells, primary rat hepatocytes (Hep), mouse fibroblasts (NIH3T3), and human adipose-derived stem cells (ADSC) were used for the spheroid culture, and the spheroid properties were compared in the Hep-spheroid, Hep/3T3-spheroid, and Hep/ADSC-spheroid conditions.
The formation of Hep-spheroid was required approximately 2 days of culture, but the spheroids formation was promoted with the presence of 3T3 cells or ADSCs. Although the 3T3 cells covered the spheroid surface, the ADSCs coagulated in the center of spheroid. Furthermore, almost same spheroid size was maintained in the co-culture conditions during culture period, but the size of Hep-spheroids decreased with the increase of culture time. The albumin secretion ability of co-cultured spheroids (Hep/3T3 or Hep/ADSC-spheroids) was higher than that of mono-cultured spheroid (Hep-spheroid), and especially the function of Hep/3T3-spheroids was highest under all conditions.
These results indicate that the co-cultured spheroids are a promising technique for the maintenance of hepatocyte functions, and the combination of cell species is important factor for modulation of cell functions.
Hydrogel materials have been widely used in tissue engineering and regenerative medicine research. For example, collagen and its derivatives are commonly used as scaffolds to incorporate cells and form 3D complex structures either by molding or 3D printing. However, these structures typically change their form dramatically in cell culture after fabrication due to cell traction forces and/or hydrogel degradation. Here, we propose a 4D (3D+time) tissue engineering approach that incorporates these changes in the design and completes the final structures during cell culture.
We encapsulated NIH 3T3 cells (RIKEN CELL BANK) in type I collagen (3 mg/mL, Nitta Gelatin Inc.) at different cell densities, and monitored the shape change for at least 3 days. Gelatin methacrylate (GelMA) was also used as a hydrogel to encapsulate cells. GelMA has emerged as a preferable hydrogel material in tissue engineering, especially for the applications of 3D bioprinting due to its photocrosslinkable and biocompatible features. By varying the UV crosslinking conditions (e.g. UV intensity and time) and GelMA concentration, as well as cell density, we observed different degrees of hydrogel shape changing during cell culture. We then fabricated bi-layer structures using two components with different shape changing properties. The bi-layer strips curved towards the side which had higher shrinking ratio (or lower swelling ratio). Their curling ratio was recorded and calculated. Furthermore, to demonstrate the capability of this 4D tissue engineering approach, we fabricated a bi-layer structure consisting 6 flower pedals, using collagen hydrogel with and without cells as the inner and outer layers, respectively. We observed the structure to form a flower shape by 窶彡losing窶・its pedals during the 3 days of culture.
In summary, our 4D tissue engineering approach can be employed to form designed structure after fabrication, and will be further explored for use of 3D bioprinting.
Chinese Hamster Ovary (CHO) cells are widely used as host cells in antibody production. The antibody production process has to aim towards high productivity and/or high titer, low operation costs and short production time. To improve productivity, cell flocculation can be applied in cell removal and/or cell culture processes to decrease impurity and turbidity. Calcium chloride is one of the cell flocculants that was studied and optimized for application [1,2]. In addition, low concentration calcium chloride is also being used as supplement in culture medium. In this study, we investigated that calcium chloride addition improved product titer, and decreased turbidity and impurities of cell culture supernatant after cell flocculation.
IgG-producing CHO-HcD6 cells were cultured as batch cultures using serum-free medium supplemented with calcium chloride at the beginning of the cultivation with 6 different concentrations (0.5, 1, 2, 5, 15 and 60 mM). Samples were recovered at the end of batch cultivation until cell viability dropped under 60 %. Cell and IgG1 concentrations during cultivation were analyzed using Vi-cell cell counter and ELISA, respectively. Dipotassium hydrogen phosphate was added at the end of cultivation, and the cells were removed from supernatants by centrifugation. Supernatants were collected for IgG1 concentration, turbidity (OD600) and host cell proteins measurements by CHO Host Cell Protein ELISA kit (Cygnus). Highest IgG1 concentration was 263 mg/L in 2 mM CaCl2. In addition, the turbidity of supernatant after centrifugation was decreased 28.2 % compared to control (without addition).
References:
[1] Chen et al., Bioprocess Biosys. Eng., 40:703–714 (2017).
[2] Burgstaller et al., J. Chem. Tech. Biotechnol., 93:1881-1890 (2018).
For medical biodegradable polyester materials to be used for soft tissue, they must be made in nonwoven or mesh form. There are many ways to make it into nonwoven form, but in order to be used for medical use, it should be a process that does not use chemical additives in manufacturing process and can produce small quantity.
In this study, various processes for the preparation of nonwoven fabrics for soft tissues were studied using biodegradable poly glycolic acid (PGA) and poly lactic-co-glycolic acid (PLGA). The web was well formed in the needle punching process. However, since the strength of the nonwoven fabric is weak and the loss of the process is large, it is considered unsuitable for medical use. The melt blown process was difficult when setting the spinning temperature during manufacture, but it was possible to produce thin, low weight nonwoven fabrics. The flat knitting process is capable of producing meshes with a small amount of 100 ~ 200g of yarn and is suitable for applying expensive biodegradable materials with little loss. In the case of the mesh fabricated by the flat knitting process, it is expected that it can be applied to the part where stretchability is needed in the future because of its excellent stretchability. Circular knitting can produce meshes with a small amount of yarn, and can be manufactured in a thin and light form, but has a disadvantage of curling the cutting portion at the time of cutting. In order to improve the morphological stability, the heat treatment process was introduced and the curling phenomenon was improved and the physical properties were also improved. We have provided a base for manufacturing nonwoven fabrics and meshes for soft tissues that can be used variously according to therapeutic purposes by using medical biodegradable polyester material.
Cataract is caused by the aggregation of crystallin proteins in lens cells of the eye, leading to light scattering and vision impairment. Cataract is known as one of the leading causes of blindness worldwide. Deposition of human γD-crystallin (HGDC), which is a major protein in eye lens, has been linked to cataract. In this study, we used the nuclear magnetic resonance (NMR) spectroscopy in combination with equilibrium chemical unfolding to investigate the structural stability of HGDC. The relatively unstable regions of HGDC were identified by comparing the residual dipolar couplings (RDCs) of HGDC in the environments without and with 5 M urea. Our results suggested that the structure of HGDC is relatively unstable in the regions including residues L92, Q113-R115, and D150-Y151. The structure of HGDC obtained by GeNMR online server was further refined using both residual dipolar couplings results and torsion angles derived from the chemical shift data. Comparison of the structures obtained from different conditions indicated that, relative to its N-domain counterpart, the C-domain of HGDC was more significantly affected by urea. Biophysical characterization of the mutants, which will be obtained by point mutation of the above suggested unstable regions, will be needed for verification.

Chinese Hamster Ovary (CHO) cells are the most commonly used host cell for the production of recombinant therapeutic proteins. In the endoplasmic reticulum (ER) of mammalian cells, ER chaperones are responsible for the folding and assembly of secretory proteins. Soluble ER chaperones have a KDEL (Lys-Asp-Glu-Leu) motif at their C-terminus that is recognized by KDEL receptors. Mammalian KDEL receptors are a family of three members that function to ensure the retrieval of ER chaperones from post ER-compartments back to the ER. In this study, we induced ER stress in CHO cells to investigate its effect on the gene expression level of KDEL receptors and soluble ER chaperones, and whether or not this response is correlated. We also hypothesize that overexpression of KDELR1 could increase the rate of retention of chaperones to the ER; therefore, increase the abundance of chaperones in ER and enhance protein folding and assembly.
The target molecules are the three KDEL receptors [KDELR1, KDELR2 and KDELR3] and KDEL chaperones [Binding immunoglobulin protein (BiP), Calreticulin, endoplasmin (GRP94), and Protein disulfide isomerase (PDI A1)]. KDEL receptors' and KDEL chaperones' gene expression during ER stress induction by tunicamycin were analyzed using Real-Time-PCR (RT-qPCR), KDEL receptors showed less than 2 fold-increase in expression level during ER stress. On the other hand, KDEL chaperones showed several fold increase. This uncorrelated upregulation proposes a possibility of the saturation of ER retention machinery (KDEL receptors), or at least hindered retention of ER chaperones under stress conditions. Recombinant stable overexpression of PDI showed increased extracellular secretion of PDI to the extracellular culture medium, indicating the saturation of ER retention machinery in CHO cells. The stable over-expression of Kdelr1 in recombinant IgG1 producing CHO cells improved IgG1 specific productivity.
Cardiac tissue engineering is an emerging field that holds great promise towards the development of innovative treatment strategies for heart disease. Nanofibrus scaffold act as a 2D surface, due to the lack of cell infiltration and hydrogels, which possess a 3D environment, suffer from lack of good mechanical properties. Therefore, by combining these two types of scaffolds, it would be possible to attain a desired 3D environment with good mechanical properties.
In this study, nanofiber-reinforced composite hydrogels were fabricated by incorporating poly (ε-caprolactone) (PCL)/gelatin nanofibers into chitosan (CS)/heart extracellular matrix (ECM) hydrogels.
To fabricate PCL/gelatin nanofibers, the dual electrospinning technique was used. To fabricate CS/ECM hydrogel, the decellularized heart tissue using appropriate combination of detergents was mixed with CS. After preparing solubilized ECM and CS solution, they were mixed in certain quantities together with specified amount of glutaraldehyde as crosslinker. Then the prepared (PCL)/gelatin nanofibers with specified size were put on the homogeneous hydrogel solution and let the hydrogel solution to penetrate into the nanofibers.
Fourier transform infrared spectroscopy (FTIR) spectra show evidence of intermolecular interactions between the CS and ECM in hydrogel.
To evaluate the physical characteristics of the fabricated nanofibers, scanning electron microscopy (SEM) and contact angle analysis were performed. The mechanical properties of the nanofiber-reinforced composite hydrogels were evaluated using Uniaxial tensile teste and the results indicated that the moduli of the nanofiber-reinforced hydrogels were remarkably higher than those of hydrogel alone. The tensile test results indicate that by varying the ratio of both hydrogel and nanofibers components, moduli between 1 to 3 Kpa and 5 to 100 Mpa could be achieved, respectively. By similar test, the native heart modulus was measured to be about 31 Kpa. By incorporating electrospun PCL/gelatin:CS/ECM (the ratio of 2:1), satisfactory mechanical properties close to that of native heart tissue were obtained.
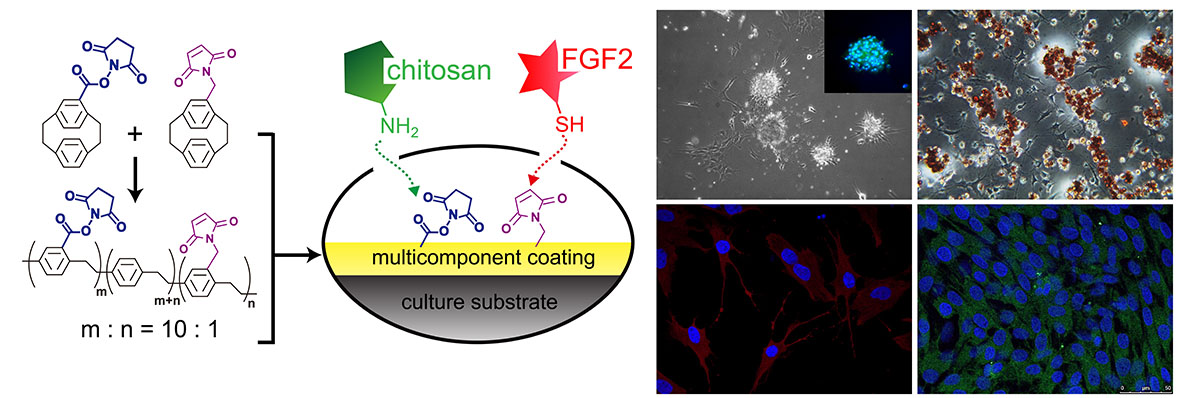
Achieving precise manipulation of stem cell self-renewal and differentiation behaviors will maximize the potential benefits of stem cell therapy. Here, the multifunctional concept of using robust and chemically defined modifications to support stem cell culture is demonstrated to provide synergistic coupling with concurrently immobilized FGF-2 and chitosan, and the resulting cell culture matrix/interface enables the proliferation of spheroids of human adipose-derived stem cells (ADSCs) with augmented stemness in the form of self-renewal and enhanced (trans-)differentiation potential for diversified cell lineages in the mesoderm, endoderm and ectoderm. The modification and the co-immobilization are performed using a straightforward one-step vapor-based coating technology applicable to a wide range of cell culture materials. The concurrent immobilization of FGF-2 and chitosan revealed an important prospective for designing a material interface that confirms that effective and sustainable factors to determine stem cell fate are obtainable using a facile multicomponent modification approach on culture substrates.