In recent years, researchers have shown an increase interest in lung disease owning to worse air pollution globally and high pulmonary attention. Several studies have examined issues related to the development of pulmonary administration of drug delivery. Yet, they still face several problems with the effectiveness of preclinical predictions on human drug responses and the shortage of high fidelity in organ-level cell testing without breathing simulation in vitro. The aim of this study was to fabricate a live and simple bronchial-like monitor with breathing motion mechanism for pulmonary drug testing. This bronchial-like monitor is regulated by deformation of the elastic membrane, leading to spontaneously air flow passing in and out. The deformation of membrane is dominated by different diameters of microchannel embedded in the bronchial-like monitor. The restriction of microchannel is contributed from the liquid flow controlled by a peristaltic pump. Therefore, the breathing mechanism can be manipulated by liquid flow rate and channel size. When liquid flow rate sets to be 0.84 ml/min (shear stress is 0.15 dyn/cm2), 328 μl/min of air flow rate and 0.49 dyn/cm2 of shear stress can be generated in the airway channel of the monitor. Additionally, the pattern of pressure drop in the airway channel also shows periodic tendency as successfully mimicking breathing motion of human. The Reynold number notes to be 0.30 that fully supports the laminar air flow in the bronchial-like airway. Furthermore, we cultured lung carcinoma A549 cells in the monitor with cyclic air flow to recapitulate the physiological relevant bronchial-like breathing model. This model, in the future, can be utilized to reconstruct the human healthy bronchiolar epithelium and to observe the toxicity and efficacy of pulmonary drug with breathing motion mechanism.
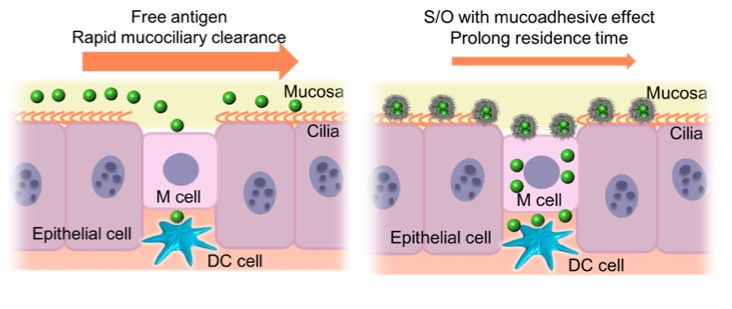
The intranasal delivery of vaccines has recently emerged as an attractive alternative to injection. The main mechanism of the intranasal vaccination is that the antigens in the vaccine transport via M cells in the nasal epithelium, then resident dendritic cells capture antigens and process them to induce the immune responses. However, it shows several disadvantages: short residence time of the antigens in nasal cavity due to the rapid nasal mucociliary clearance and weak immune responses due to the inefficient antigens transportation to the immune system.
To overcome these issues, a Solid-in-Oil (S/O) nanodispersion technique was developed for intranasal vaccine delivery. It is based on nanodispersions of hydrophilic antigens coated with hydrophobic surfactant molecules in an oil vehicle. Surfactant molecules and the oil vehicle could increase the nasal residence time of the antigens due to its mucoadhesive effect. Moreover, nanosized particles is expected to improve the delivery efficiency of the antigens to the immune system and induce strong immune responses (Fig.1).
We investigated the potential of intranasal vaccination by using S/O nanodispersions loaded with a model antigen ovalbumin (OVA). S/O nanodispersions containing OVA in different oil vehicles were prepared and the formulation using squalene oil was chosen due to the small particle size. Moreover, nasal absorption and nasal residence time of OVA were assessed to be increased by S/O nanodispersions. Finally, the OVA-specific antibody levels from serum and nasal mucosal washes from the mice were evaluated after intranasal vaccination. We found that intranasal administration of S/O nanodispersions induced strong mucosal and systemic immune responses and could be used for the future intranasal vaccination.
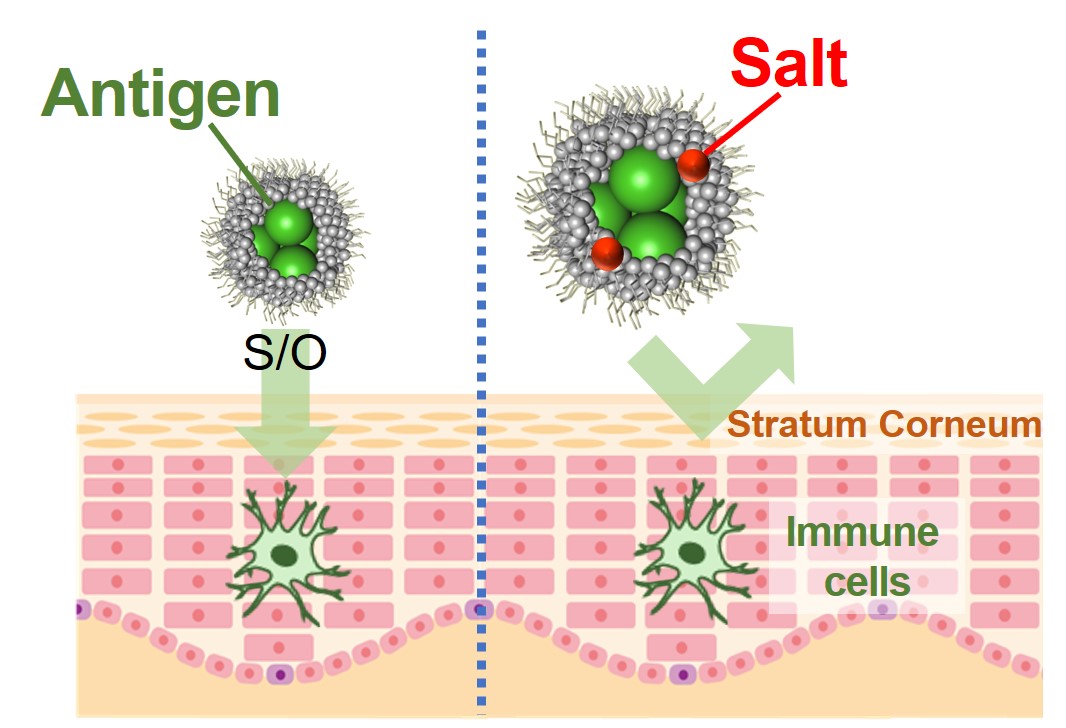
Transdermal delivery has been developed as one of the most attractive administration methods of vaccine antigen, which is safe and convenient. In transdermal vaccination, we need to deliver antigen to the immune cell in the skin to start the immune response. However, it is difficult for hydrophilic macromolecules such as protein to be permeated into the skin due to hydrophobic barrier function of stratum corneum; the outmost layer of skin. In recent years, it has been reported that skin permeability of the antigen is enhanced by using a solid-in-oil (S/O) nanodispersion, in which hydrophilic substances were coated with a hydrophobic surfactant and dispersed in oil as nanometer-sized particles. The purpose of this research is to prepare a practical transdermal vaccine by S/O technique from commercially available injection vaccine, in which antigen is dissolved in saline because osmotic pressure of injection vaccine is adjust with body fluid. In this study, fluorescein isothiocyanate-labeled ovalbumin (FITC-OVA) and isopropyl myristate (IPM) were used as a model antigen and an oil base with skin penetration effect, respectively. To check the effects of salts on nanoparticle formation and skin permeability, FITC-OVA was initially dissolved in saline according to commercially available vaccine and prepared S/O nanodispersions. As a result, it was confirmed that the particle diameter increased and the skin penetration amount decreased in the sample of high salt concentration. It is shown that it is important to reduce the salt concentration to make a transdermal vaccine from injection one.
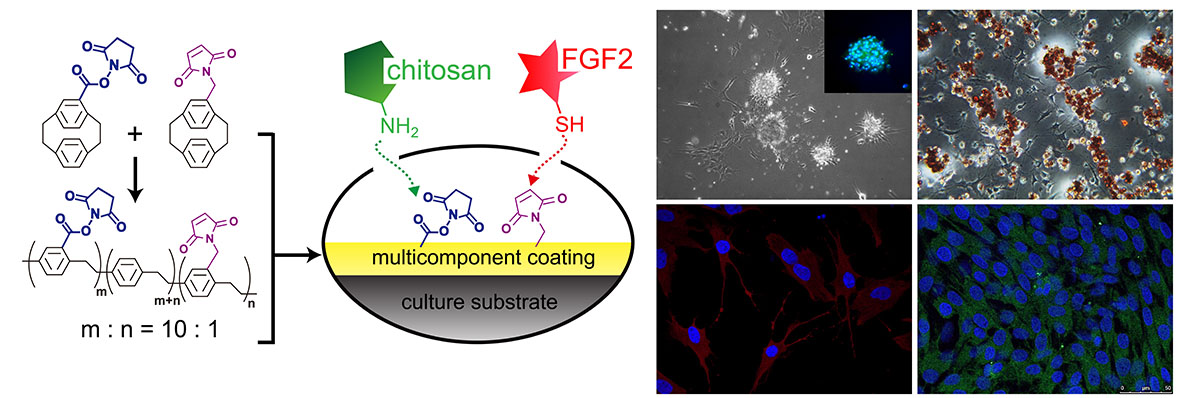
Stem cells are attractive source for tissue engineering applied on regenerative medicine, translational medicine, and drug discovery. Batch type culture is the typical way for stem cell culture, which is laborious and expansive. Moreover, the digestive enzymes or EDTA solutions to detach cells are causing cell damage and increasing production costs. For reducing the costs, we developed the continuous culture system culturing the human embryonic stem cells (hESCs) on thermoresponsive polymer surfaces (Fig. 1). hESCs could be detached from the thermoresponsive polymer surfaces by reducing the medium temperature below the lower critical solution temperature (LCST) of the thermoresponsive polymer (poly-N-isopropylacrylamide and its copolymer). After reducing the temperature, the thermoresponsive surface will become more hydrophilic, creating an unfavorable environment for cell attachment. Thus, hESCs can be partially detached by gentle pipetting. The remained cells could be confluent again by adding fresh medium. This continuous culture system could be used for 3D-cultivation. However, the barrier of applying this system is hESCs became harder to attach on the thermoresponsive surfaces coating with the extracellular matrix (ECM) after multicycles. This maybe cause by degradation of ECM after long-term culture. Therefore, we investigated the precoated and uncoated methods with different ECM; recombinant vitronectin (rVN) and recombinant laminin-511 (iMatrix-511). Comparing with traditional precoated method, the uncoated method where ECM is added into culture medium upon seeding cells, provides cost-effective and time-efficient method and maintains hESCs pluripotency during long-term culture. This uncoated method applied to continuous culture system saves processing time and cost and contributes to the regenerative medicine.
Transdermal delivery has been developed as one of the most attractive administration methods of peptides, which are used as biopharmaceuticals for refractory diseases or synthetic antigens for vaccination including cancer treatment or prevent from infections. In transdermal vaccination, we need to deliver peptide antigen to the immune cell in the skin to start the immune response. However, it is difficult for hydrophilic macromolecules such as peptides to be permeated into the skin since stratum corneum; the outmost layer of skin shows the hydrophobic barrier function. In this study, we developed oil-based formulation of peptides mediated by ionic liquid (IL) in order to achieve the efficient skin permeation. We used cytotoxic T cell epitope peptide (OVA 256-264, sequence: SIINFEKL) as a model peptide. This peptide is hydrophilic and not soluble in isopropyl myristate (IPM), which is an oil base with skin penetration effect. To disperse the peptide in IPM, the ILs consisting of choline cation and fatty acids anion were synthesized. These ILs are biocompatible and show high affinity to oil because they are derived from natural molecules and fatty acids have long alkyl chains. We successfully dispersed the hydrophilic peptide in IPM by adding the ILs and ethanol as co-solvent. Subsequently, we checked whether the peptide in oil formulation shows skin permeability and the permeation mechanism of peptide. The peptide with ionic liquid in oil formulation permeated into the model mouse skin, while the penetration of peptide in aqueous phosphate buffer was low. Moreover, we revealed that this ionic liquid in oil acts as the skin permeation enhancer by extracting and disordering the lipid in the stratum corneum. These results suggested that oil-based formulation using biocompatible ILs is a promising strategy for transdermal peptide delivery.

Vaccines are the most useful means for preventing infection, in which antigens are mainly administered by injection, and induce a systemic immune response in whole body to eliminate pathogens. In the case of an oral vaccine, antigens are captured M cells and delivered into Peyer's patches, lymph node-like lymphatic tissues, by the transcytosis, followed by the presentation of mucosal immunity. The ability of oral vaccines to activate mucosal immunity is attractive compared to injection because it is possible to prevent the initial infection. In this study, we tried to develop an oral vaccine delivery using a solid-in-oil (S/O) nanodispersion, in which hydrophilic drugs are coated with hydrophobic surfactants and dispersed in oil phase as nanoparticles. In addition to prevention of antigen degradation by low pH or enzymes, it is expected that the antigens are effectively delivered to the lymphatic tissues using oil-bases because hydrophobic materials such as lipids are known to be formed micelles and absorbed by lymphatic tissues rather than blood circulation. The S/O nanodispersion was prepared using ovalbumin (OVA) and sucrose fatty acid esters as a model antigen and surfactants, respectively. From in vitro release study using artificial intestinal fluid containing lipase and bile acids, the S/O nanodispersion using sucrose laurate (L-195) and perilla oil showed higher antigen release, while that using sucrose erucate (ER-290) and squalene oil released antigens gradually, resulted from the length of alkyl chain of surfactants and ester bonds of oils. From the oral vaccine study in vivo, the OVA-specific IgA production in the small intestine of mice was increased by the S/O nanodispersion using ER-290 and squalene oil compared to aqueous vehicle, phosphate buffered saline (PBS). These results suggested that the S/O nanodispersions releasing antigens gradually is suitable to deliver antigens to Peyer's patches, and induced mucosal immunity effectively.

Introduction.
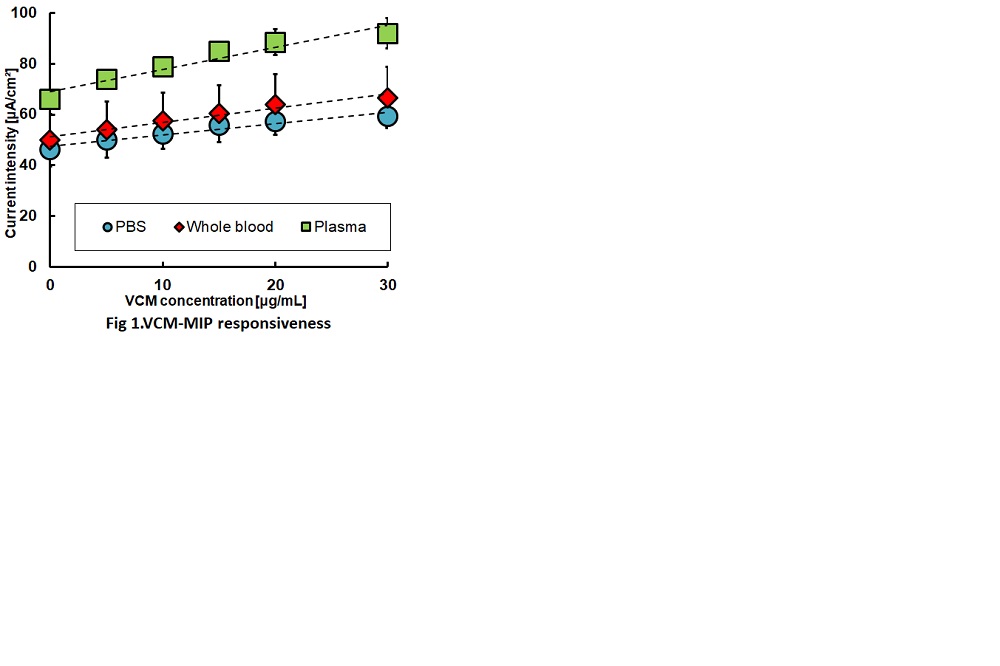
Vancomycin is the drug of first choice for treating Methicillin-Resistant Staphylococcus Aureus (MRSA) infections which is the main causative bacteria of nosocomial infection. Therapeutic drug monitoring (TDM) of VCM is strongly recommended in order to prevent side effect and creation of the resistant bacteria. However, TDM does not work sufficiently, because frequent measurement of blood level of VCM is still difficult. We developed a blood-VCM sensor with a paste electrode of graphite particle on which a molecularly imprinted polymer (MIP) is immobilized covalently in this study. The sensitivity of the sensor to the VCM concentration in whole blood and plasma was evaluated.
Experimental method.
Graphite particles were coated with photoinitiator of radical polymerization was dispersed in a polymerization solution and irradiated with xenon lamp light. The MIP carbon was mixed with silicone oil and filled in a glass tube to prepare a carbon paste electrode immobilizing MIP of VCM.
The analyte solvent was prepared by dissolving VCM in phosphate buffer, bovine whole blood or plasma, and the relationship between current obtained by differential pulse voltammetry and the VCM concentration was observed.
Results and Discussion.
The calibration line of the VCM-MIP electrode is compared in each analyte solution at Fig. 1. First, the electrode indicated sensitivity to VCM in all analyte solutions. However, the sensitivity in plasma is about twice as high as in PBS or whole blood. It can be thought he detection of VCM is not interfered by blood cells. But oxidation of uric acid, or other kind of oxidative species in plasma enhances background current and oxidative current of vancomycin. But the enhancement is probably suppressed by the blood cells. However, the result indicates that the VCM-MIP electrode enables the sensing of VCM in whole blood without separation between the plasma and blood cells. The property is advantageous for real time monitoring of VCM.
Human pluripotent stem cells (hPSCs) includes human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) are a promising source for regenerative medicine and tissue engineering. Typically, hPSCs are used to culture on the extracellular matrix (ECM) coated dishes, such as Matrigel, recombinant vitronectin (rVN) and laminin-521 (LN-521), which were secreted from animal or human cells. However, those ECMs provided uncertain chemical composition. Therefore, the xeno-free synthetic biomaterials offer not only a chemical defined composition but also a reproducible culture condition.
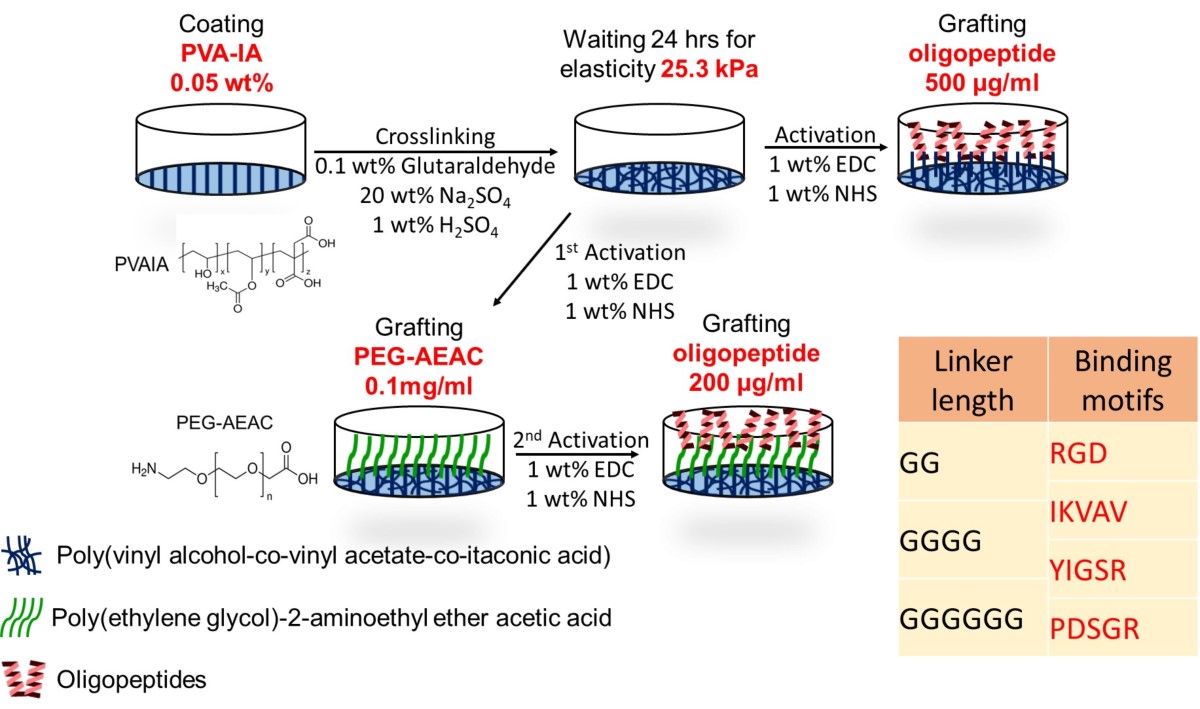
In our previous study, we successfully cultured hPSCs on polyvinylalchohol-co-itaconic acid (PVA-IA) hydrogels, with optimal crosslinking elasticity, conjugated with vitronectin-derived oligopeptides (KGGPQVTRGDVFTMP), which provide RGD binding motifs for cell adhesion. The further key cell binding mechanism will be investigated by using PVA-IA hydrogels grafted with synthetic peptides from different chain compositions. Those synthetic peptides chains contain five variants of α-chain, four variants of β-chain and three variants of γ-chain of laminin, respectively. These synthetic peptides will be investigated by comparing the ability to support long term hPSCs culture and differentiation into cardiomyocytes (CMs). Therefore, we prepared PVA-IA hydrogels grafted with different oligopeptide sequences derived from Laminin α-chain (RGD), α1-chain (IKVAV) and β1-chain (YIGSR and PDSGR) to compare the efficiency of cell attachment and differentiate into CMs (Fig. 1). We also design some motifs with different length of joint segment to mimic the original ECM and evaluate the length effect on the culture of hPSCs. In this study, the expression four pluripotency gene (Oct3/4, Klf4, Sox2, and Nanog) of hPSCs is evaluated for ability to support hPSCs growth. hPSCs are induced into cardiomyocytes to screen the optimal synthetic peptide. We expected to screen the optimal combination of synthetic peptides for hPSC culturing and differentiation.
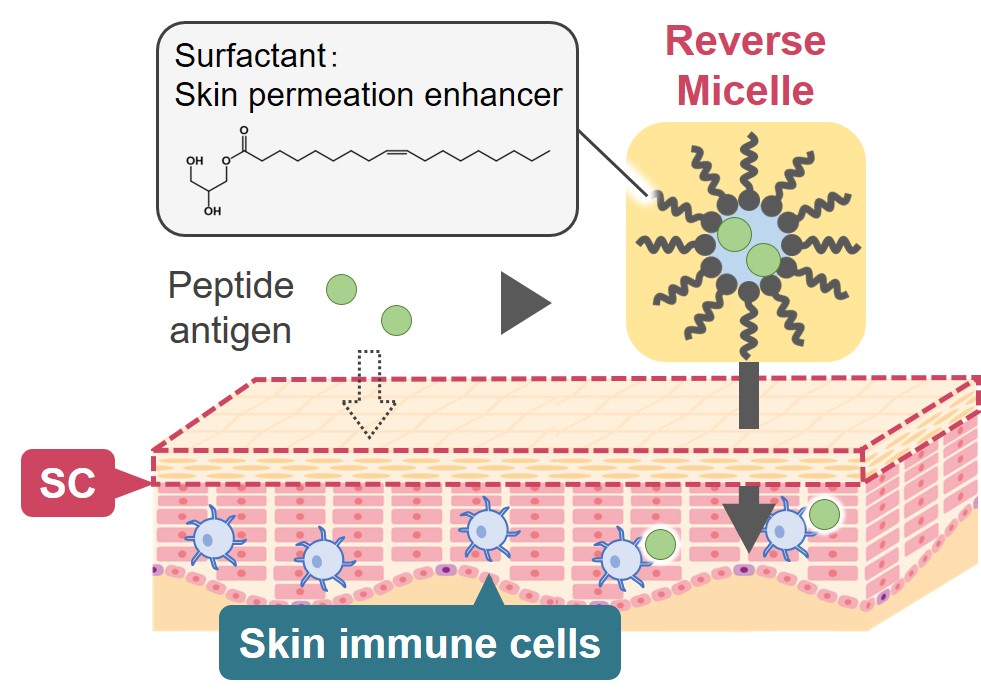
Cancer vaccine, a prophylactic therapy against cancer by administering a cancer antigen to activate the host immunity, has come to draw a great deal of attention due to its low risks of side effects. In this study, we focused on the transcutaneous drug delivery system for the cancer antigen delivery. This delivery route would be the ideal way, because skin immune cells are known to induce a strong immune response. However, the stratum corneum (SC), the outermost layer of the skin, works as a hydrophobic barrier to prevent the penetration of antigens which are mostly hydrophilic. To overcome this problem, we tried to use Reverse Micelle Formulation (RMF). In RMF system, hydrophilic drugs are enclosed inside the aqueous core of reverse micelles and dispersed in oil phase which SC prefers. Despite there were numerous reports regarding transcutaneous delivery using RMF, there was no report on a transcutaneous vaccine due to its still low skin permeability. In this study, we used amphiphilic Skin Permeation Enhancer (SPE) as surfactant which forms reverse micelles to realize even higher permeability. To the best of our knowledge this is the first report on this strategy, and we conducted experiments to prove its effectiveness.
We chose K-TRP-2 (KKKGSVYDFFVWL) peptide as a cancer antigen, and fatty acid glycerol ester as amphiphilic SPE. By dropping the aqueous solution of antigen onto surfactant-containing oil, we obtained uniform RMF. As a result of in vitro experiment, RMF showed a significant improvement in skin permeation of antigens. Also, RMF enabled the successful antigen delivery to the skin immune cells, which is a key step to induce an immune response, and its transcutaneous treatment recruited more cytotoxic T lymphocytes into tumor microenvironment than an injection treatment and strongly suppressed a tumor growth.
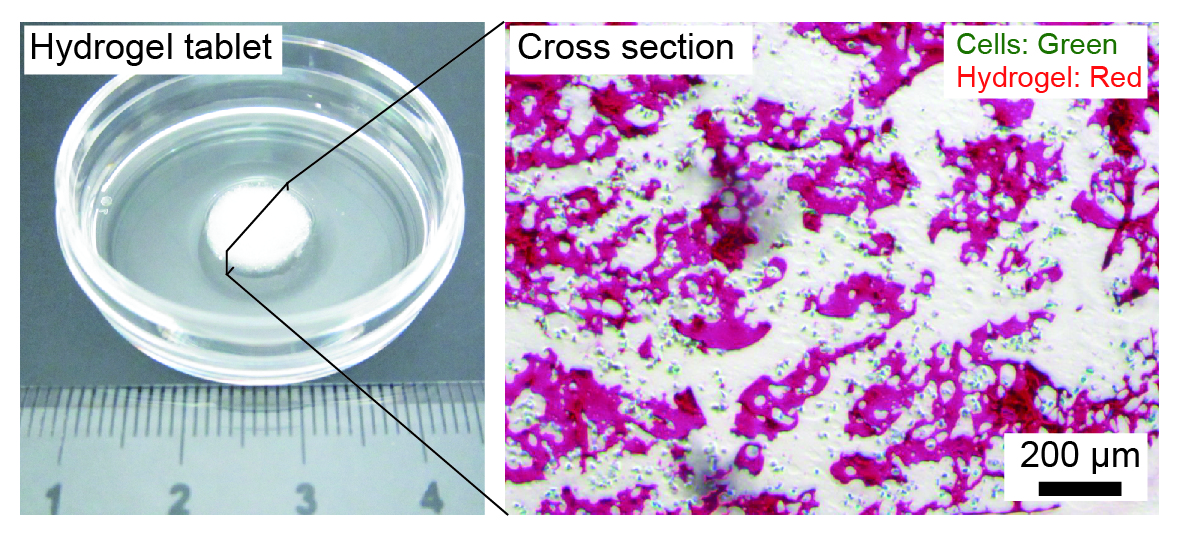
Preparation of three-dimensional (3D) organ models composed of liver cells is expected as an efficient approach to in vitro cell-based drug screening and development. Various types of hydrogel-based 3D cell culture systems have been developed, but the lack in the capillary networks in hydrogel matrices causes severe problems in terms of insufficient supply of oxygen and nutrition. Here we proposed a facile strategy for creating perfusable hydrogel-based liver cell culture systems. We utilized a bicontinuous aqueous two-phase dispersion, which was composed of polyethylene glycol (PEG)-rich and gelatin methacrylate (GelMA)-rich phases, to produce cell-encapsulating microporous gelatin-based hydrogels. The interconnected pores were utilized to perform perfusion culture of encapsulated HepG2 cells.
We first prepared an aqueous two-phase system of PEG/GelMA, and then remixed the separated two phases by controlling the mixing ratio, with the addition of a photopolymerization initiator and cells. Immediately after mixing, the GelMA-rich phase was photocrosslinked by irradiating UV light, to form millimeter-sized tablets of microporous sponges. We confirmed that most of the encapsulated cells were viable during this mixing and crosslinking processes, and after cultivation for 1 week. In contrast, cells encapsulated in uniform GelMA hydrogel were mostly dead after 1 day of cultivation. The expressions of hepatocyte-specific genes were upregulated for the hydrogel sponges, which was confirmed by RT-qPCR. Additionally, we performed perfusion culture of the cells, by packing the obtained hydrogel sponges into microfluidic perfusion chambers, and evaluated the functions of the encapsulated cells. The presented hydrogel sponges are useful because we did not need any complicated devices or protocols for preparing hydrogel sponges, and they would be applicable as a unit platform for organs-on-a-chip systems.
In this study, we report synthesis of hydropholic surface and active-target superparamagnetic iron oxide nanoparticles (SPIONs) for hyperthermia cancer therapy. Superparamagnetic iron oxide nanoparticles (SPIONs) capping oleic acid were prepared by thermal decomposition method. The synthesized iron oxide nanoparticles were encapsulated with the polyaspartamide (PA) to enhance their biocompatibility and hydrophilicity (PA-encapsulated SPIONs). A polysuccinime (PSI) which is biocompatible and biodegradable is the backbone of PA. In addition, multifunctional polymers including hydrophilic O-(2-aminoethyl) polyethylene glycol (PEG) and hydrophobic octadecylamine (C18) were grafted on PSI for amphiphilic structure, and on biotin for increasing the cancer cellular uptake. The diameter of the PA-encapsulated SPIONs was confirmed by TEM and DLS. The structure of the polyaspartamide was confirmed by 1H NMR. The structure and biomedical properties of PA-encapsulated SPIONs were investigated in vitro and in vivo experiments.

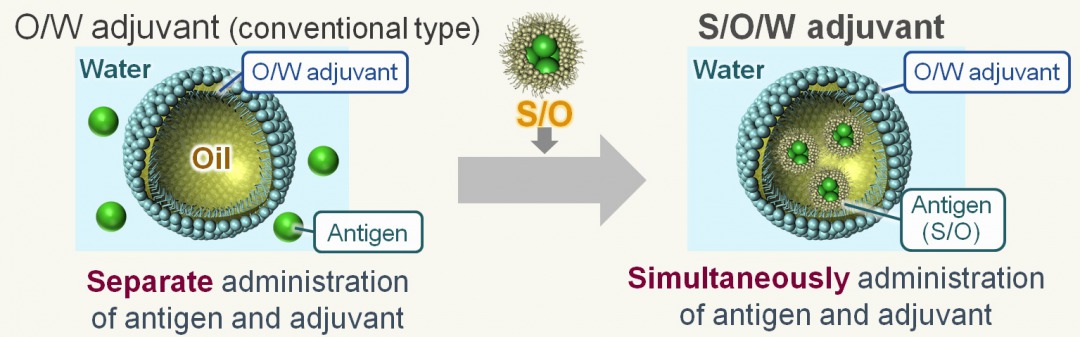
A vaccine is a drug that administers an antigen derived from a pathogen to the body and induces antigen-specific immunity. In the case of therapeutic vaccines, it consists of antigens and adjuvants (additives to enhance the immune response), and various delivery systems that deliver them to immune cells have been reported. In several studies, strong vaccine effects were induced using intelligent lipid or polymer-based delivery systems. However, most of the candidates have not been put to practical use because novel, synthesized compounds must be confirmed the safety in human. Mostly, it takes over 10 years to accept to use clinical phase, and it is hard to balance high safety and effect of vaccine delivery. To overcome this problem, we used a clinically accepted adjuvant, oil-in-water (O/W) emulsion, as vaccine carrier. Though it has high safety and effect for human, the efficiency is limited because hydrophilic antigen and O/W emulsion are administered separately. In this study, we tried to create a solid-in-oil-in-water (S/O/W) adjuvant that encapsulates antigens in oil phase using solid-in-oil (S/O) technology and can simultaneously administer antigen and adjuvant. Composition of S/O/W adjuvant is based on MF59, commercially available O/W emulsion, and ovalbumin (OVA) was used as a model antigen. The S/O/W adjuvant was determined as a nano-sized emulsion (ca. 300 nm) and over 90 % of antigen was encapsulated after 24 h. From in vitro study using dendritic cells, efficient intracellular delivery and lysosomal escape of antigens were achieved by S/O/W adjuvant. Furthermore, when S/O/W adjuvant was administered subcutaneously to mice in vivo, the antigen-specific humoral and cellular immunity was induced more effectively than O/W emulsion and OVA free. These results suggested that S/O/W adjuvant, which can achieve co-delivery of antigen and adjuvant, is a novel approach to develop effective carriers for therapeutic vaccine such as cancer immunotherapy.
Virus-like particles (VLPs) can be produced using recombinant DNA technology by expressing viral surface proteins that spontaneously assemble into particulate structures similar to authentic viral particles. VLPs serve as safe and effective vaccines and diagnostic antigens. The baculovirus–insect cell system has been used extensively for the production of a wide range pf VLPs. However, the baculovirus system has several inherent limitations including contamination of VLPs with progeny baculoviruses. Stably transformed insect cells can be used as attractive alternatives to the baculovirus–insect cell system. In the present study, the production of influenza VLPs by recombinant insect cells was investigated.
The DNA fragments encoding hemagglutinin (HA) and matrix protein 1 (M1) of an influenza virus A (H1N1) were individually cloned into the plasmid vector pIHAbla and pIHAneo. The pIHAbla and pIHAneo contained the Bombyx mori actin promoter downstream of the B. mori nucleopolyhedrovirus (BmNPV) IE-1 transactivator and the BmNPV HR3 enhancer for high-level expression, together with either a blasticidin or a neomycin resistance gene for use as a selectable marker, respectively (Yamaji et al.: Biochem. Eng. J., 41, 203–209 (2008)). After cotransfection with the resultant plasmids, Trichoplusia ni BTI-TN-5B1-4 (High Five) cells were incubated with blasticidin and G418, and cells resistant to the antibiotics were isolated.
Western blot analysis of a culture supernatant showed that transfected High Five cells secreted HA and M1. Sucrose density-gradient sedimentation analysis and dynamic light scattering of the culture supernatant suggested that secreted HA and M1 molecules were produced in a particulate form. Hemagglutination assay using chicken erythrocytes revealed hemagglutination activity in the culture supernatant. Taken together, recombinant insect cells may offer a promising approach to the development and production of influenza VLPs.
Mesoporous silica nanoparticles (MSNs) conjugating doxorubicin (DOX) via a pH-sensitive cleavable linkage, hydrazine (HYD) were synthesized. MSN-HYD-DOX were encapsulated with the polyaspartamide (PASPAM) grafted with the hydrophilic o-(2-aminoethyl)-o′-methylpoly(ethylene glycol) (PEG) and the cell permeating ligand, biotin (Biotin). The chemical structure of the synthesized MSN-HYD-DOX and PASPAM-g-PEG/Biotin was confirmed using FT-IR and 1H-NMR spectroscopy. The mean diameter of the MSN-HYD-DOX@PASPAM-g-PEG/Biotin nanoparticle was 142 nm and 121 nm, respectively, examined by dynamic light scattering (DLS) and transmission electron microscope (TEM). The HYD bond was effectively cleaved in acidic condition, and thus DOX was released much faster at pH 5.0 than at pH 7.4. The cell viability in MSN-HYD-DOX@PASPAM-g-PEG/Biotin system was much lower than that of the free DOX drug because of efficient intracellular drug delivery associated with the biotin ligand.
Gene delivery methods for animal cells are one of the most important tools in biotechnology fields such as gene therapy, generation of transgenic animals and pharmaceutical protein production. We have attempted to develop a gene transfer method based on retrotransposon. Retrotransposons are mobile elements that transfer themselves in a cell genome by the “copy-and-paste” mechanism. The process is known as retrotransposition. A retrotransposon, long interspersed element-1 (LINE-1, L1) is categorized as a non-LTR retrotransposon, comprised of a 5' untranslated region (5'UTR), two non-overlapping open reading frames (ORFs) separated by a short inter-ORF sequence, and a 3'UTR that ends in an adenosine-rich tract. We constructed an L1-based vector plasmid including the essential components for retrotransposition. An intron-disrupted Neor reporter gene and a scFv-Fc expression unit under the control of CMV promoter were inserted into 3'UTR region in order to evaluate retrotransposition and scFv-Fc production. CHO-K1 cells transfected with the plasmids were screened using G418 for 10 days. The established cell clones produced scFv-Fc proteins in the culture medium. To control retrotransposition, we removed the ORF2 sequence from L1 vector to generate ORF2-deleted L1 (L1[$Delta;orf2]) vector, and constructed ORF2 expression vectors (pORF2 and pORF1-2) for providing ORF2 proteins. Under the optimal ratio of L1[$Delta;orf2] vector and ORF2 expression vector, no significant difference in the number of G418-resistant colonies formed was observed compared with that for L1 vector although it slightly decreased. The scFv-Fc gene was detected in all clones by genomic PCR. Real-time PCR revealed that scFv-Fc gene was integrated into the genome at the range from one to six copies in the clones.
Stem cells are an attractive prospect for regenerative medicine and tissue engineering. However, stem cell are difficult to differentiate into specific cell types. Thus, reliable methods to differentiate stem cells into desired cell types are necessary to be developed. Here, we developed nanosegment-grafted biomaterials having different elasticity. [1] We also developed optimal differentiation media to induce the differentiation of hES cells into cardiomyocytes. We developed several biomaterials having different elasticity for hPSCs differentia-tion into cardiomyocytes. We prepared (1) ECM (extracellular matrix)-coated dishes, (2) PVA-IA (polyvinylalcohol-co-itaconic acid) hydrogel dishes having different elasticity that are grafted with several ECMs [2], and (3) PVA-IA hydrogel dishes having different elasticity that are grafted with cell-adhesion oligopeptide (oligovitronectin). We also investigated the differentiation efficiency using different induction medium: (a) Commercial cardiomyocyte induction medium, (b) RPMI 1640 medium supplemented with B27 and bovine serum albumin (BSA), (c) CDM3 medium (xeno-free culture medium) developed by literature [3] and in this study. On day 0, we replaced the expansion medium into cardiomyocytes differentiation medium containing the GSK3β inhibitor. On days 1-2, we observed that 30%~40% of the cells in the medium shown in medium (a) and 50~60% of the cells in the medium (b) were died and detached from the surface. However, the center of the colony of living cells were getting thicker and became compact. These cells were differentiated into cardiomyocytes on days 5-6. On day 8-10, we successively observed the contracting colonies on each biomaterial surface. We successfully screened the optimal elasticity of bio-materials, preferable nanosegments immobilized on the biomaterials as well as the cell culture medium for the optimal differentiation of hPSCs into cardiomyocytes. This system will be used for developing for cell sorting system, which will be a great benefit to its clinical application in regenerative medicine.
For acute and fulminant liver failure patients, liver transplantation is only an effective treatment. However, it has a big problem in donor organ shortage. As a bridge use for liver transplantation, bio-artificial liver (BAL) systems with extracorporeal blood circulation have been an attractive choice. For constructing BAL systems in which functional hepatic cells are filled in a bioreactor, hepatoma cells derived from liver carcinoma are a candidate cell source due to their high proliferative capacity, although liver function of hepatoma cells is considerably lower than that of primary hepatocytes. In our previous study, genetically engineered mouse hepatoma cells with inducible high liver function were established by transducing liver-enriched transcription factor (LETF) genes (Yamamoto et al., Biochem. Eng. J., 2012, 60, 67-73; J. Biosci. Bioeng., 2018, 125, 131-139). In this study, human hepatoma HepG2 cells were genetically engineered to express liver function in response to heat treatment. For this purpose, we constructed a heat-inducible expression of LETF genes, in which a tetracyclin-responsive artificial transactivator is expressed under control of a heat-shock protein promoter with transcriptional amplification and the transactivator then induces LETF genes. The gene expression system was constructed on transposon vectors and introduced into HepG2 cells. Transgenic cells (HepG2/HSP/8F) were screened by drug (puromycin and blasticidin) selection. For inducing overexpression of LETF genes, cells were heated at 43 °C for 30 min using water bath. Albumin secretion and cytochrome P450 activity were measured for the cells with or without heat treatment. The liver functions of HepG2/HSP/8F cells were enhanced by the heat treatment. The cells with heat-inducible liver function can be a new cell source for various hepatic studies including construction of BAL systems.
Encapsulation of water-insoluble bioactive compounds using chitosan-oleic acid complex particle was investigated. One-step preparation of chitosan-oleic acid complex particles encapsulating bioactive compounds with a narrow diameter distribution was carried out by the simple mixing of a chitosan aqueous solution and an ethanol solution containing oleic acid at room temperature. The formation of particles with mean diameter below 1 micrometer was confirmed by dynamic light scattering and laser diffraction measurements. A characteristic peak indicating aggregation of alkyl chain of oleic acid molecules was observed by small angle X-ray scattering measurement, suggesting that the particle had hydrophobic domains formed by aggregation of oleic acid molecules inside the complex particles. These characteristics agreed with scanning-transmittance electron microscopic images of complex particles. Various water-insoluble compounds including curcumin, capsaicin, and vitamin E were successfully encapsulated into the complex particles. Diameter distribution of the complex particles encapsulating bioactive compounds were maintained during storage over a month, suggesting that the particles were highly stable. The particle suspension could be concentrated by evaporation and powderized by freeze-drying without significant changes in their diameter. The results demonstrated that our method for encapsulating water-insoluble hydrophobic bioactive compounds using chitosan-oleic acid complex particles were potentially applicable as edible materials for development novel food dispersion systems.
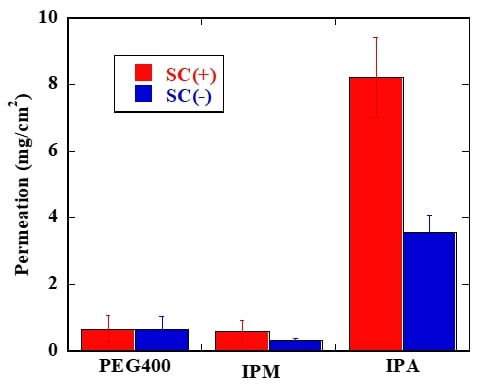
A local anesthesia (LA) relieves the sense of a specific part of the body. It is used in the medical procedure for needle puncture or venous cannulation. The main administration of LA is injection, but this method is not only invasive and painful but also requires specialized knowledge and risk of secondary infection, so an alternative administration is required. Transdermal delivery of LA has advantages that enable to bring anesthesia effect without any pain and difficulty. The transdermal LA is available in commercial, but the current marketed product is not popular because it cannot eliminate pain completely and needs long-term continuous administration. Therefore, we aim to improve the effect of transdermal local anesthesia using non-aqueous base materials. Previously, the relationship of the pharmacokinetics and pain-relieving effect of LA are not revealed completely. These facts make difficult to improve analgesic effectiveness of LA. In this study, we conducted early clinical study in human and compared the results obtained in clinical trials with in vitro studies in laboratory scale. We used lidocaine as LA, and polyethylene glycol 400 (PEG400), isopropyl myristate (IPM) and isopropyl alcohol (IPA) as the base. In clinical study, we found that LA using IPA induced actual analgesic effectiveness and achieved painlessness within 10 minutes. On the other side, we found that IPM showed high permeation across the pig skin in vitro. It is suggested that high permeability across the skin did not lead improving analgesic effectiveness. Subsequently, we measured the amount of drug in the skin, and the sample using IPA as the base showed highest amount of lidocaine in the skin (shown in Figure 1, SC: stratum corneum). It is suggested that staying drug in the skin leads improving actual analgesic effectiveness because the result of clinical study corresponds with the result in vitro.
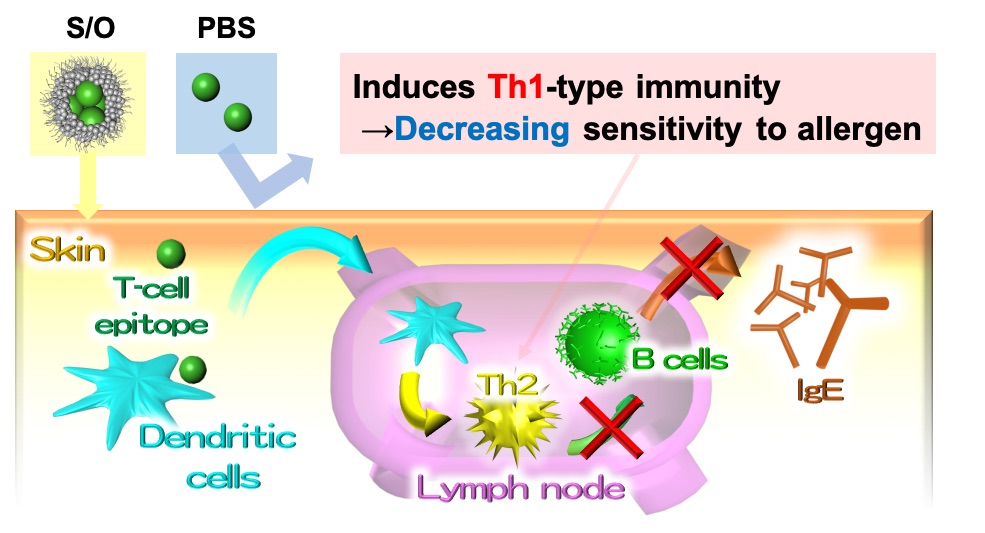
In recent years, the number of Japanese cedar pollinosis patients are increasing, and pollinosis immunotherapy has attracted much attention. However, the administration of a whole pollen allergen by injection has the risk of side effects such as anaphylactic shock. In this study, we tried to develop a simple, non-invasive method of pollinosis immunotherapy by the way of transcutaneous administration and used cedar pollen T cell epitope peptide A (PepA: SMKVTVAFNQFGP) with a low risk of adverse effects as an antigen. The presence of stratum corneum (SC) which is the hydrophobic barrier in the outermost layer of the skin makes it difficult for the hydrophilic antigen to penetrate the skin. To overcome the barrier, antigens were dispersed in hydrophobic solvent as nano-order particles using the solid-in-oil (S/O) nanodispersion, in which antigens are coated with hydrophobic surfactant molecules, to permeate through the SC. In this study, K-PepA, in which hydrophilic trilysin was conjugated to PepA, was used to increase the solubility in water and stability of the S/O nanodispersion.
First, the mice were vaccinated with K-PepA thrice with a one-week by injection. One week after the final immunization, splenocytes were harvested and T cells were quantified using flow cytometry. Similar to PepA, an increase in the number of Th1 cells was observed by K-PepA, suggesting the efficacy for pollinosis immunotherapy. Second, the S/O nanodispersion was obtained by freeze-drying the water-in-oil emulsions containing K-PepA in water phase and surfactant, a sucrose laurate in oil phase, and dispersing it in isopropyl myristate. The permeability of K-PepA enclosed in S/O nanodispersion to mouse skin was evaluated in vitro and a significant improvement in permeability was confirmed as compared with the PBS solution. These results suggest that the S/O nanodispersion containing K-PepA is a promising approach to effective transcutaneous pollinosis immunotherapy.
Transdermal drug delivery system (TDDS) is a drug administration method that has attracted attention in terms of safety, convenience, and low invasiveness. However, it is difficult for hydrophilic micromolecular drugs to be delivered into the skin surface since the stratum corneum, which is the outermost layer of the skin, has a hydrophobic barrier function. Therefore, it is effective to use a method of dispersing drugs in an oil base having a transdermal penetration promoting effect, but hydrophilic drugs are not soluble in oil base. In this study, the use of ionic liquids (ILs) to solubilize poorly soluble substances was considered. It is important that ILs are low toxicity and consist of biocompatible materials. So far, choline has been used as a cation in many reports on the application of ILs to TDDS. In this presentation, we synthesized a new biomaterial-based IL using ethyl esterified β-alanine as the cation, and compared with a choline-based IL. In both ILs, oleic acid was used as the anion, which is expected to have high affinity to oil base. We selected ovalbumin epitope peptide as a model drug. The peptide is hydrophilic and was insoluble in oil base, isopropyl myristate (IPM), but the addition of the β-alanine-based IL succeeded in dispersing the peptide in IPM. On the other hand, the peptide is not clearly dispersed in a simple mixture of IPM and choline-based IL. Furthermore, when the permeability of the peptide to pig skin in vitro was evaluated, it was hardly penetrated by the aqueous phosphate buffer solution because of stratum corneum's hydrophobic barrier function, but showed high permeability by the solution dispersed in IPM using β-alanine-based IL. These results suggest that the combination of a new biomaterial-based IL using amino acid as the cation and IPM is a useful strategy for TDDS.
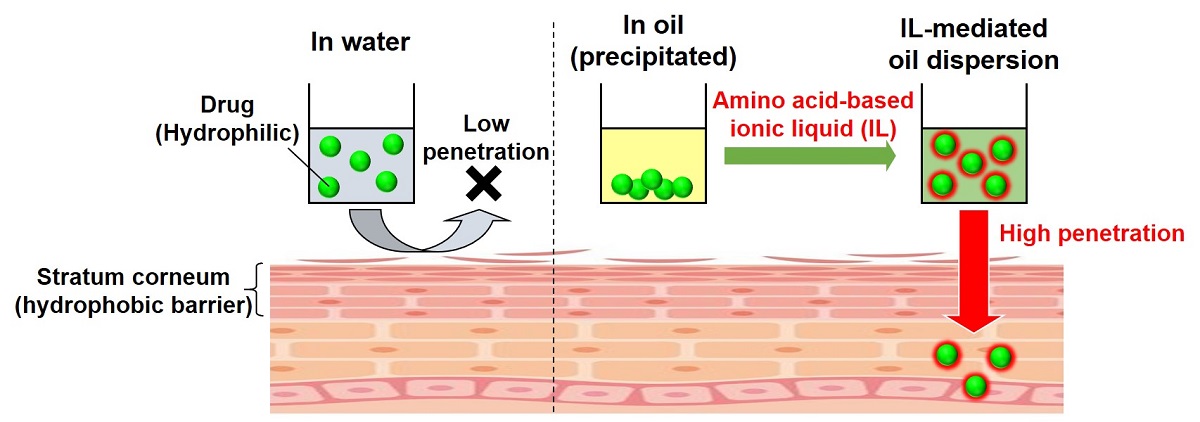
Vaccine is the most effective way to prevent infection by administrating antigen to enhance immunity. Currently, most vaccines are administrated by subcutaneous injection, but it has some problems. Therefore, transcutaneous immunization has attracted attention as a new vaccination method. Transcutaneous immunization is a novel vaccination strategy that delivers antigens to intact skin topically to induce protective immune responses. This needle-free strategy is easy-to-use, safe, and low invasive compared with conventional subcutaneous injection. The starting point of transcutaneous immune mechanism is a capture of antigen by dendritic cells (DCs) in the skin. DCs as a strong immune response inducer, could be expected by the transcutaneous route. However, the skin surface is covered with hydrophobic stratum corneum, which make it difficult to deliver hydrophilic antigens to DCs. To overcome this difficulty, we developed a novel formulation of nano-dispersed solid-in-oil (S/O). The novel S/O formulation can be prepared only by high-speed stirring, and it can contain various additives in large amounts. In this study, we tried to improve the vaccine effect by two approaches of promoting skin permeation and activating immune cells, by adding skin permeation enhancer and adjuvants to the novel S/O formulation.
On the study of antigen uptake by DCs, after 12 hours of vaccine administration to mice, skin cells were analyzed by flow cytometer. As a result, the novel S/O formulation containing skin permeation enhancer and adjuvant significantly increased the antigen delivery rate to the skin DCs, it is 4 times of aqueous solution. In the evaluation of vaccine efficacy, blood antibody level after vaccine administration was measured. As a result, the novel S/O formulation induced antibody production equivalent to injection.
In conclusion, by adding skin permeation enhancer and vaccine adjuvants to the novel S/O formulation, we succeeded the development of transcutaneous vaccine which has an effect comparable to injection.

In recent years, biopharmaceuticals have attracted much attention. This is because they are considered effective for diseases that cannot be treated with conventional small drug molecules. Therefore, there is a need to establish a useful method for administering biopharmaceuticals to patients. At present, administration methods for such biopharmaceutical are limited to injection, because oral administration degrades protein molecules, which are the main active ingredients of pharmaceuticals, by digestive enzymes. On the other hand, injection administration is less convenient because it requires a medical doctor, and it often involves the risk of needle stabbing and pain. In the present study, we aim to establish a simple and safe administration method to replace injection and focus on transdermal administration. For the purpose, we need to overcome the serious problem of transdermal administration, which is a strong barrier of skin surface for transdermal delivery of biomolecules. In this research, we proposed a novel drug carrier called “reverse micelles” for the transdermal drug delivery. Reverse micelles are oil-based molecular assembly, in which various kinds of biomolecules can be entrapped into the water cores. Focusing on the fact that the skin surface is hydrophobic, we thought that the entrapped biomolecules in reverse micelles could permeate into the skin. This reverse micellar method has been used for small molecule drugs so far, however it has not been applied to large biomolecules such as peptides or proteins. In this study, we have developed reverse micelle formulation that enables successful transdermal delivery of biomolecules, and discussed the permeation mechanism by reverse micelles.

In the production of pharmaceuticals, xenoantigens derived from animal cells and animal-derived materials may be present in the final products. These compounds are often problematic and must be identified. Among them, xenoantigen carbohydrates have immunogenic effects and difficult to remove from drugs because they bind to proteins.
Anti-αGal is a natural antibody in humans. Binding of this antibody to αGal antigens (αGal epitope) expressed on porcine xenograft surfaces is a major factor affecting engraft survival. Recently, studies showed that some therapeutic antibodies and cell processing materials used in reproductive medicine contain the αGal epitope. Another major xenoantigen is N-glycolylneuraminic acid (Neu5Gc). An enzyme is responsible for Neu5Gc synthesis; when the gene encoding this enzyme is mutated, humans cannot synthesize Neu5Gc. Humans produce antibodies against Neu5Gc glycan as heterogenous structures, causing the immunogenicity of therapeutic proteins containing Neu5Gc glycan epitopes. Therefore, rapid and robust methods for detecting the αGal epitope and Neu5Gc are required. We chemically synthesized various structure-defined N-glycans including human-type glycans (G0, G2, and SG), uniform isomers (6-G1 and 3-G1) and heterogenic antigens including αGal or Neu5Gc. We also produced high-grade 2-AB-labeled N-glycans. These compounds are suitable for mass spectrometry, capillary electrophoresis, and high-performance liquid chromatography analyses. We simultaneously developed polyclonal antibodies that specifically bind to xenoantigen carbohydrate, αGal, and Neu5Gc using chicken. Some antibodies were modified with fluorescent probes or enzymes for versatile detection.
In this poster, we introduce new methods for detecting xenoantigen carbohydrates in cell culture supernatants or purified antibodies with our reagents. Xenoantigen carbohydrate attached to Fc and/or Fab regions of the antibody can be detected using our methods. We also introduce a method for detecting xenoantigens in the cell culture supernatant in the early stages of cell establishment.
Biological membranes are dynamic and heterogeneous lipid bilayer structures involved in numerous cellular processes, not only as simple boundary but also active uptake of external molecules. A new approach, termed as membrane-lipid therapy, has proved the possibility that some of lipophilic molecules can show therapeutic effect as like drug, by interacting with biomembrane. Therefore, the alteration of lipid membrane properties has been expected to promise a novel and great potential of therapeutic effect. Here, a self-assembled pro-drug system have been investigated, focusing on controlling the self-assembly behavior by surroundings such as pH. Pro-drug structures were inspired from self-assembling fatty acids (FAs). FAs are amphiphilic molecule with hydrophobic carbon chain and hydrophilic carboxyl group, whose phase states strongly depend on pH condition and molecular structure. The aryl carboxylic acids Oxaprozin (Oxa), a main kind of nonsteroidal anti-inflammatory agent, was chosen as model molecule and designed to Oxa-lipid prodrug molecules possessing carboxyl group with different carbon chain length and achieved control of drug self-assembly behavior under different pH conditions. Designed pro-drug molecules achieved the control of drug self-assembly behaviors based on pH condition, which could realize the development of biological microenvironment-sensitive drug delivery system. It can be expected that the variation of self-assembly structure might contribute to enhanced therapeutic efficiency.
Reference: P.V. Escriba et al., Trends Mol. Med., 12, 34 (2016). Han J. et al., J. Phys. Chem. B, 121, 4091 (2017). M. Bucciantini et al., FASEB J., 26, 818 (2012).

Supramolecular polymers based on molecular self-assembly has attracted attention in fabricating bio-functional materials because of design diversity and biomimeticity in structural and functional aspects.[1] Self-assembling peptide amphiphiles (PAs) undergo spontaneous supramolecular assembly in aqueous solutions and have shown a potential in the use in biomedical applications, such as drug delivery carriers. In this study, we developed a novel co-assembly system based on PAs to fabricate a carrier that enables the control of assembled structures. To encapsulate small molecular drugs into PA nanomaterials, we introduced a complementary hydrogen bonding interaction into PAs and drug derivatives. Cyanuric acid (Cya) and melamine (Mel) derivatives are known complementary hydrogen bonding pair, which forms 3:3 network structure called rosette, thus we synthesized Cya-conjugated PAs (Cya-PAs) and Mel-attached model drug (Mel-NBD). To vary the assembled structures to study the influence of the structural aspects on the efficiency in intracellular drug delivery, several hydrophobic substituents of Cya-PAs were introduced; from C0 (Cya-PA1), C6 (Cya-PA2), to C12 (Cya-PA3). When Cya-PAs and Mel-NBD were mixed in a 1:1 molar ratio, they formed distinct nano-assemblies. Interestingly, the morphologies and dimensions of the assemblies were highly dependent on the PA design; i.e. globular objects (~100 nm) were observed with Cya-PA1, whereas fibrous assemblies were obtained with Cya-PA2 (2-300 nm) and Cya-PA3 (> 1 μm). In vitro study on intracellular delivery of Mel-NBD using HeLa cells showed that the cellular internalization efficiency increased in the form of assembly compared with Mel-NBD alone. Moreover, the cellular uptake was differed by the size of the carrier. These results indicate that this co-assembly system provides controllable nanostructures for an efficient delivery of small molecular drugs.
[1] R. Wakabayashi et al., Chem. Commun., 2019, 55, 640–643.
Metabolism and differentiation of culture cells are influenced by changes in cellular morphology, cell-cell and/or cell matrix interactions, and medium components, and so on. To control the destiny of cells, it is important to understand the relationship between these parameters and cell properties. In this study, we focused on the effects of cellular morphology on cell properties. Monolayer culture has been widely used as a conventional culture technique. On the other hand, a spheroid (spherical organoid) culture has been established as typical three-dimensional culture technique. In this study, we investigated the difference of cell proliferation and neuronal differentiation of NT2 cells, model cell of neural research, in the monolayer and spheroid cultures.
For the spheroid culture, we fabricated the microwell chip which consisted of 195 circular microwells (600 μm in diameter) on a poly-methylmethacrylate plate, and the surface was modified with polyethylene glycol to promote spheroid formation. Traditional tissue culture plate was used for monolayer culture. NT2 cells were cultured in a medium containing retinoic acid (RA), an inducer of neural differentiation.
In the monolayer culture, the cells adhered and extended on the plate. In the spheroid culture, the cells were aggregated in each microwell and formed the spheroid within 24 hours of culture, and the spheroid morphology was maintained during culture period. Although RA treatment suppressed the cell proliferation in both culture systems compared to RA non-treatment condition, the cell proliferation ability in monolayer culture was higher than that in spheroid culture. The neuronal differentiation ability of NT2 spheroid culture was higher than that of monolayer culture, and the differentiation ability was dramatically enhanced by RA treatment.
These results indicate that the proliferation and differentiation properties of NT2 cells are influenced by the difference of cell morphology.
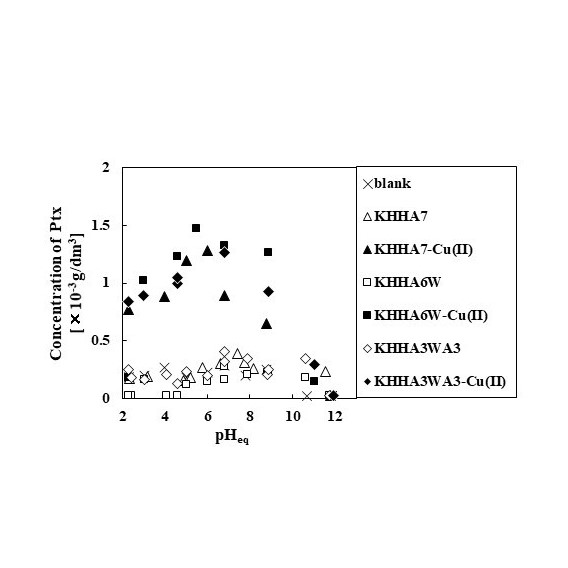
Stimuli-responsive drug delivery systems have attracted attention because of their potential to maximize therapeutic effects and minimize side-effects. Recently, authors have developed an amphiphilic short-chain peptide (KHHA7), which consists of a hydrophobic domain based on seven alanine residues and a hydrophilic domain based on a lysine and two histidine residues (KHH), as a dispersant for a poorly water-soluble anticancer drug paclitaxel (Ptx). As the histidine residues form a stable chelate complex with intermediate metal ions such as Cu(II) and Ni(II), the dispersibility of the complex between Ptx and KHHA7 alters in the presence or absence of metal ions. In the present study, two analogs of KHHA7 (KHHA3WA3 and KHHA6W), in which one of the alanine residues in KHHA7 was substituted to a tryptophan residue, were prepared to investigate the effect of substitution on the dispersibility of the complex.
The amphiphilic peptides were chemically synthesized by Fmoc solid-phase synthesis method. The products were purified using HPLC and identified by MALDI TOF-MS. The complex between peptides and Ptx was prepared by mixing an aqueous solution containing the peptides as well as Cu(II) and an ethanol solution of Ptx, followed by removal of ethanol in vacuo and lyophilization. To the complex was added 0.10 mL phosphate buffer solution to evaluate of the apparent solubility of Ptx. When Ptx was complexed with the peptide alone, the dispersibility of Ptx was not significantly different from that of the blank sample. The apparent solubility increased with increasing the concentration of Cu(II). The dispersibility of Ptx complex using KHHA3WA3 and KHHA6W was comparable to that using KHHA7, suggesting that the effect of substitution to tryptophan on the complex stability is small.
Mesenchymal stem cells including adipose-derived stem cells (ADSCs) are expected as a cell source for tissue engineering and regenerative medicine, because they can be differentiated to osteoblasts, chondrocytes, adipocytes, and so on. 2D monolayer culture that cells are extended on a culture substrate has been widely used as a conventional culture technique. On the other hand, 3D culture, such as spheroid (spherical organoid) and cell-sheet, has attracted attention as a new culture technique. In this study, we investigated the difference of ADSC properties in the monolayer (2D) and spheroid (3D) cultures.
As a spheroid formation platform for 3D culture, we fabricated a microwell which consisted of 195 circular microwells (400μm in diameter) on a poly-methylmethacrylateplate, and the surface was modified by polyethylene glycol to promote the spheroid formation. Traditional tissue culture plate was used as a monolayer culture platform for 2D culture. The cell proliferation and differentiation properties of ADSCs were compared between monolayer and spheroid cultures.
In the 2D culture, the cells adhered and extended on the culture plate, and proliferated during culture period. In the 3D culture, the cells were aggregated in each microwell and formed the spheroid within 6 hours of culture. The spheroid morphology was maintained in each microwell. However, the size of ADSC spheroids was almost same during culture period, and the cell proliferation of spheroid culture was suppressed than that of monolayer culture. The expression levels of differentiation marker genes (osteoblast, chondrocyte, and adipocyte differentiations) in the spheroid culture were higher than those in monolayer culture.
These results indicate that the proliferation and differentiation of ADSCs are influenced by the difference of cell morphology, and the spheroid culture is a promising method to promote the cell differentiation.
In development processes of anti-cancer drugs, cell-based assays have been used as one of drug screening techniques. Generally, monolayer culture of cancer cells has been utilized as in cancer model, but it is difficult to mimic morphology and environments of in vivo neoplasm. On the other hand, cancer cell spheroids, which are formed by the rearrangement and compaction of cell aggregates, have attracted attention as more useful solid cancer model. We have developed a spheroid culture platform, microwell chip, which can achieve size control, easy medium change, and spheroid mass production. In this study, we investigated the difference of drug responses of cancer cells in the monolayer and spheroid cultures.
The microwell chip consisted of 200 circular microwells (600 μm in diameter) on a poly-dimethylsiloxane plate, and the surface was modified by polyethylene glycol to promote the spheroid formation. Traditional tissue culture plate was used as a monolayer culture. HCT116 cells (colorectal cancer cell line) and doxorubicin (anti-cancer drug) were used as the cancer model cell and model drug, respectively. The cells were treated with different doxorubicin concentrations, and the changes in cell morphology and cell survival were evaluated in the both culture platforms.
HCT116 cells formed a single spheroid in each microwell within 3 days of culture. The spheroid growth was inhibited by the doxorubicin treatment, and the collapse phenomenon at outside region of spheroids occurred by the doxorubicin treatment of high concentration. In the monolayer culture, the cells were detached from culture plate with the increase of doxorubicin concentration. The cell survival rate against doxorubicin in spheroid culture was higher than that in monolayer culture, indicating that HCT116 spheroids have high drug resistance than monolayer culture.
These results suggested that spheroid culture has a potential as cell-based assay technique for drug screening.
Hybrid liposomes (HL) composed of L-α-dimyristylphosphatidylcholine (DMPC) and polyoxyethylene (25) dodecyl ether (C12(EO)25) were effective for therapeutics and detection for tumor of orthotopic graft model mouse of breast cancer.
In the therapeutic effects of HL for breast cancer, high inhibitory effects of HL without drugs on the growth of breast cancer (MDA-MB-453) cells were obtained along with apoptosis in vitro. Membrane fluidity of MDA-MB-453 cells treated with HL was significantly increased as compared with that of control and DMPC liposomes. We examined therapeutic effects of HL for orthotopic graft model mice of breast cancer after the inoculation to mammary gland. Reduction of tumor in the group treated with HL was observed, although enlargement of tumor in control and DMPC groups was confirmed on the basis of volume, weight and autopsy. Many apoptotic cells were observed in the tumor section of orthotopic graft model mice of breast cancer treated with HL using TUNEL method.
With regard to detection (diagnosis) on breast cancer using HL, enhanced selective accumulation (diagnosis) of HL/ICG including a fluorescence probe (Indocyanine green; ICG) only toward breast cancer cells having high membrane fluidity without affecting normal breast cells was confirmed in vitro. Enhanced accumulation of HL/ICG into the tumor of orthotopic graft model mice of breast cancer was observed into the tumor of orthotopic graft model mice of breast cancer was observed. These data indicate that HL accumulated in tumor cells in the mammary gland of the orthotopic graft mouse model of breast cancer for a prolonged period of time, and inhibited the growth of MDA-MB-453 cells.
Therapeutic effects by HL and detection (diagnosis) by HL/ICG of cancer with enhanced tumor accumulation for orthotopic graft model mouse of MDA-MB-453 for the use of HL as theranostics agents were revealed for the first time in vivo.
Specific binding on liposome of the trehalose with a high hydration power has been reported. Trehalose liposomes (DMTre) have been produced by using sonication of a mixture of L-α-dimyristoylphosphatidylcholine (DMPC) and α-D-glycopyranosyl-α-D-glucopiranoside monomyristate (TreC14) in buffer solutions with no organic solvent. Inhibitory effects of trehalose liposomes on the growth of tumor cells along with apoptosis have been reported. Growth inhibition against colon, gastric, hepatocellular and leukemia cells has been obtained using trehalose liposomes without drugs.In this study, we investigated inhibitory effects of trehalose liposomes on the proliferation of human breast adenocarcinoma ( MDA-MB-453 ) cells were examined in vitro and in vivo. DMTre with a hydrodynamic diameter less than 100 nm, were preserved for 4 weeks. The inhibitory effects of DMTre on the proliferation of MDA-MB-453 cells accompanied with apoptosis were obtained after the enhancement of accumulation of DMTre into MDA-MB-453 cell membranes. An increase in membrane fluidity of MDA-MB-453 cells treated with DMTre was observed on the basis of fluorescence depolarization method. DMTre suppressed the activity of NFκB. DMTre caused apoptosis for MDA-MB-453 cells through the activation of caspases and mitochondria. Suppression on the filopodia formation of MDA-MB-453 cell by DMTre was observed using confocal laser microscopy. Inhibitory effects of DMTre on the invasion of MDA-MB-453 cells was also obtained using invasion assay. Tumor weights on the xenograft model mice of MDA-MB-453 intravenously administered with DMTre markedly decreased as compared with those of the control group. Brown color indicating apoptotic cells was observed in many tumor cells of tissue section of tumor in xenograft model mice after the treatment with DMTre. DMtre suppressed metastasi in hepatic metastasis mouse model of breast tumor after the intrasplenic inoculation of MDA-MB-453 cells. Therapeutic effect of DMtre were also obtained using hepatic metastasis mouse model of breast tumor.
Among various drug delivery systems, micro/nanocarriers consist of liquid metal are being actively studied. Eutectic Gallium-Indium alloy (EGaIn) liquid metal has been reported to have a low melting point, low toxicity, and proper tissue/cell penetration. EGaIn has a characteristic property, deforming by the control of light and heat irradiations. In this study, we describe transformable liquid metal nanoparticles delivering anti-tumor drug molecules. These “nanotransformer” particles are synthesized through sonication, and extrusion of either EGaIn solution or EGaIn mixed with amphiphilic lipids. The resulting nanotransformers are loaded with an anti-tumor drug, doxorubicin that can be released upon deformation of EGaIn nanoparticles excited with light. The nanotransformer particles showed no toxicity in mammalian cells. A proper photo-thermal ligand added to the nanotransformer helped to increase the yield of deformed liquid metal nanoparticles, resulting in efficient drug delivery.
A phospholipid forms a self-assembled membrane such as micelles, bicelles and vesicles to reduce an exposure of hydrophobic chain groups in aqueous solution. Interestingly, bicelles are disk-shaped aggregates composed of long-chain phospholipids that make up a planar region and either detergent or short-chain phospholipids that compose flanking rims. They have an advantage over micelles in their ability to mimic natural membranes and, therefore, capture membrane proteins in their biologically relevant orientation as demonstrated in structural studies of the transmembrane segment, functional studies of membrane protein and drug delivery system.
Previously, we reported that as “bicelles”, are made of comprise a mixture of long-chained phospholipids (e.g., 1,2-dimyristoyl-sn-glycero-3-phosphocholine, DMPC) and short-chained phospholipids (e.g., 1,2-dihexanoyl-sn-glycero-3-phosphocholine, DHPC) at high concentration in aqueous solution, wherein DHPC detergents cover the rim of DMPC bilayer. In addition, bicelles can be reformed vesicles depend on a various condition such as total lipid concentration and DMPC/DHPC fraction ratios. However, it is very difficult to control of vesicle size by using simple flow microfluidic chip.
In this study, we demonstrate simple method to control the size of vesicles through dilution of bicelles solution by using microfluidic chip which has integrated with passive micromixer and microchannel structure to produce the flow focusing. The results shows that the final production is formed vesicle structure of ordered phase through fusion of bicelles. In addition, we verified the membrane properties with the membrane fluidity (1/P_DPH), polarity (GP340) and optical density (OD500). Our novel microfluidic chip has potentials to create a lipid vesicles which can be controlled size by using total flow rate ratio and DMPC/DHPC fraction ratio.

Quantum dots (QDs) are promising fluorescent probes for many biological applications because they have superior and unique optical properties in comparison to conventional organic dyes. Traditionally, covalent coupling is employed to immobilize or conjugate antibodies onto QD surface for preparation of QD-antibody immunoprobes. In this study, we proposed a simple and effective technique to conjugate CdTe QD with immunoglobulin G (IgG) antibody using high-affinity bifunctional peptides. First, five CdTe QD-binding peptides were obtained from phage display screening. To identify the shortest amino acid sequence needed for the QD-binding activity, the screened peptides were subjected to truncation assay using SPOT-peptide array. Linking the truncated peptides with an antibody Fc domain-binding peptide1 was later done to design mediate bifunctional peptides for bioconjugation of CdTe QD with IgG antibody. The affinities of bifunctional peptides to CdTe QD and IgG antibody were confirmed by surface plasmon resonance (SPR) system. Due to no extensive surface preparation steps and chemicals as covalent conjugations, the designed bifunctional peptides suggest simply and biological-friendly conjugation process of CdTe QD with IgG antibody. Our peptide-mediated molecule conjugation strategy would be beneficial in development of QD and antibody-based molecular imaging.
1Sugita, T. et al. Screening of peptide ligands that bind to the Fc region of IgG using peptide array and its application to affinity purification of antibody. Biochem Eng J. 2013, 79, 33–40.
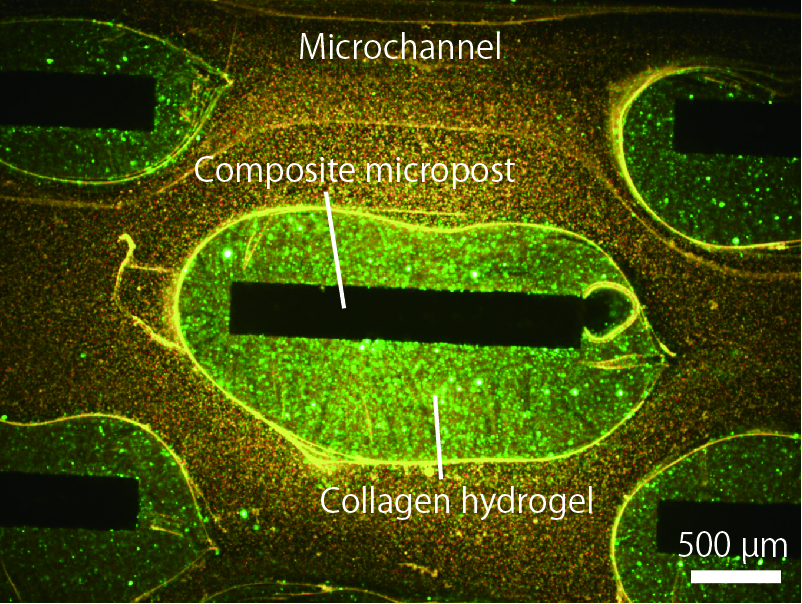
Engineering of collagen-based hydrogels in microfluidic devices is a promising technology for cell culture experiments with well-controlled environmental parameters. However, it is not a trivial task to integrate microfabricated, cell-encapsulating hydrogels into microfluidic devices. Here we present a new approach to precisely engineering type-I collagen-based hydrogels in microfluidic devices, with the help of gelation agent-incorporating composite silicone substrates. We created microposts composed of polydimethylsiloxane (PDMS) and phosphate salt (Na2HPO4) powders, which work as a neutralizer for acidic collagen solution into collagen hydrogels selectively around the microposts. It was possible to encapsulate living mammalian cells into the hydrogels, which could be extended to perfusion culture of cells for organs-on-a-chip applications.
In the experiments, we fabricated PDMS microfluidic devices equipped with microposts with various morphologies, by soft lithography and replica molding techniques. After introducing an acidic collagen solution into the channel and incubating the solution for several minutes, washing buffer was introduced to remove the non-gelled collagen solution from the channel. As a result, we successfully fabricated collagen hydrogels selectively around the microposts (see the attached figure). Additionally, we were able to create vascular tissue models, when we employed a microchannel whose lumen was completely made of the composite PDMS substrate containing phosphate salt powders. Living mammalian cells could be encapsulated in the hydrogel matrices while keeping high cell viability, showing its applicability to perfusion cell culture. The presented strategy would be highly useful because we could fabricate collagen hydrogel-based microstructures in microfluidic devices without the need for complicated devices or operations.
Neonatal hyperbilirubinemia, which is also called neonatal jaundice is a common symptom in the newborn. Bilirubin accumulates in the newborns' blood and high concentration of bilirubin is potentially toxic to the central nervous system, so it's critical to monitor the level of bilirubin. Current bilirubin diagnosis is still based on diazotization reaction and calibrated with the standards using an automated clinical chemistry analyzer to obtain the bilirubin value. The total measuring time is about 30 min. However, most of the diagnosis has to be completed in large hospitals or medical centers due to the costly diagnosis equipment. For those who lives in remote areas or are lack of medical resources, it's impossible to obtain immediate medical diagnosis.
In this work, we combine the advantages of paper-based diagnosis technology, the efficiency of the active separation process, and the aspects of the microfluidic platform to construct a bilirubin diagnosis chip which can measure the values of total, direct and indirect bilirubin. The diagnosis time of bilirubin chip can be controlled within 5 min and the requirement of sample volume is only one drop of the blood. The samples can be quantitatively analyzed by using the bilirubin chip directly and the colorimetric readout which can be obtained through a smartphone camera without using the expensive and bulky equipment. Moreover, we will establish a standard operating procedure for neonatal bilirubin testing and apply it directly to clinical trial. After the comparison of the clinical chemistry analyzer and transcutaneous bilirubin meter, we can establish a real time, easily operate, and precise bilirubin testing system to meet the goal of point-of-care diagnosis.
Mesenchymal stem cells (MSCs) have been ideal candidates for cell therapy because of extensive proliferative ability and stemness. We tried to use nonwoven fabrics (NWF) as a cell culture scaffold. NWF has three-dimensional fiber aggregates formed by heat bonding, which has a high surface area for cell adhesion and elongation. Although NWF have been reported to be a candidate scaffold for large scale expansion of MSCs, quality of cells grown in NWF was not known well. In this report, MSCs grown in a disc scaffold of NWF for 3 weeks showed higher osteogenic differentiation potential and percentage of CD90 positive cells, compared with MSCs grown in a dish bottom surface. Amount of extracellular matrix (ECM) per unit surface area of fibers was larger than that on a bottom surface of dish at first 2 weeks of culture. The osteogenic differentiation potential of the MSC inoculated onto cell-free ECM-coated dish was higher as the amount of ECM increased. Consequently, the higher percentage of CD90 positive cell and higher osteogenic differentiation potential of cells grown in a NWF disc than cells cultured on a dish might at least partly due to the higher amount of ECM.
Keywords: mesenchymal stem cell, nonwoven fabric, ECM, CD90, osteogenic differentiation
Nanostructured multi-phase lipid career (NMLC) is recognized as potential delivery vehicle for sensitive biological compounds. It has been reported that the drug release behavior from NMLC is affected by the type of lipids, emulsifiers and conditions employed for preparation. In this study, multi-phase lipid particles were prepared by hot shear homogenization method with wax and triglyceride mixture. This particulate system is designed to release compounds dissolved in the triglyceride phase by the reaction of intestinal enzyme (i.e. lipase). The carnauba wax, distilled water and coconut oil were homogenized (15,000 rpm, 100°C, 5 seconds) and cooled rapidly at 0°C. Samples with different ratio of coconut oil and wax, addition of emulsifier (i.e. sorbitan monooleate, sodium cholate), and different cooling temperature (i.e. 0°C and 30°C) were prepared. The particle size and surface structure were observed with a scanning electron microscope (SEM). The NMLC dispersion was reacted with simulated intestinal fluid (i.e. lipase, maleic acid buffer (pH 6.5), 37°C). After the reaction, the amounts of the free fatty acids (FFA) produced by the coconut oil were measured by neutralization titration. The particle size of the prepared NMLCs was in the range of 2–100 μm. Formation of the channel of coconut oil could be observed on the surface of the particle. When these NMLCs were applied to the intestinal fluid, FFA were successfully released from the NMLCs. The released amounts of FFA were similar for the samples without emulsifiers. This was because the coconut oil phase exposed on the particle surface did not differ largely among the samples. The amounts of FFA formation were greatly increased in the samples with the emulsifiers. It found that the emulsifier would help the lipase reaction, which accelerates the hydrolysis of coconut oil.
Availability of quartz crystal microbalance (QCM) technique as screening method of drug–loaded ceramic material was examined. Hydroxyapatite {apatite; Ca10(PO4)6(OH)2} was coated on a QCM sensor with wet synthesizing or self-assembled monolayer (SAM)-mediating procedure. Adsorption and desorption behavior of two anticancer reagents: mitoxantrone dihydrochloride (MXT) and doxorubicin hydrochloride (DOX) were followed on the QCM sensor-supported apatite. The 80 μL reagent of 1g/L-distilled water was injected into 8 mL water-containing glass cup where the sensor tip had been immersed. The decreased frequency (ΔFdec) in injection was about 300 for MXT or 500 Hz for DOX, which indicated adsorption of both reagents occurred in agreement with the order of the adsorbed amounts on apatite particle.
After he frequency (F) became constant for about 1 h, phosphate buffered solution (PBS, pH 7) of 100 mmol/L was injected. MXT and DOX showed opposite profiles. For MXT, the F increased in injection, indicating that desorption occurred possibly due to substituting MXT by ions in PBS on the surface. On the contrary, the F for DOX did not increase but did decrease more after the constant F, i.e., adsorption in pseudo-equilibrium. This result was much different from that for apatite particle, in which desorption of DOX was clearly observed in a batch system. Dependency of the injected concentration of PBS (0 – 100 mmol/L) on the ΔFdec was examined. As the concentration increased, the ΔFdec became larger, suggesting that PBS facilitated adsorption of DOX as opposed to MXT.
The Zeta potentials of MXT and DOX were much different: MXT was almost zero while DOX was highly-positively charged: about 23 mV. From these results, we considered that negatively–charged phosphate ion in PBS bound to positively-charged DOX and then less-charged DOX adsorbed on the surface, with possibly weak binding such as van der Waals adsorption.
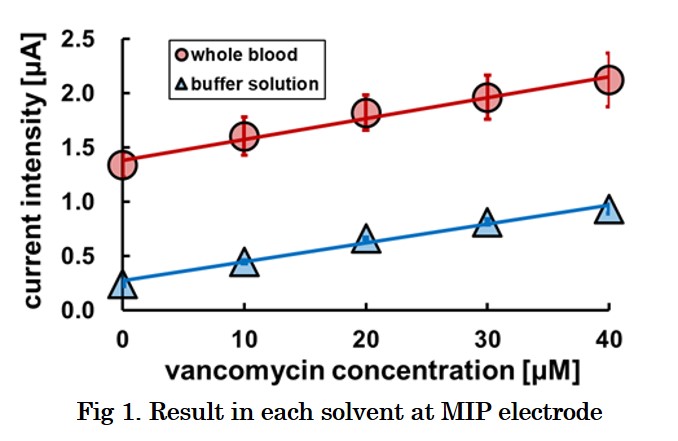
Vancomycin is the first-line antibacterial agent against methicillin-resistant Staphylococcus aureus (MRSA). Overdose of vancomycin leads to kidney damage, but underdose allows creation of the resistant bacteria. Therefore, therapeutic drug monitoring (TDM) is strongly required for chemotherapy with vancomycin. However most of hospitals outsource the analysis of the drug concentration in blood. It takes 2-3 days to obtain the data of the blood level, but there is no guarantee that the appropriate administration is done during the days. Then, we are developing a real-time vancomycin sensors using molecularly imprinted polymers (MIP). MIP is a synthetic polymer which memorizes structure of a target molecule in its matrix during its synthesis. Although their function resembles antibody but MIP can be obtained more easily and economically because their materials are same as familiar plastics. We are developing a vancomycin sensor by fixing MIP on the indium tin oxide (ITO) electrode surface. We investigated the polymerization conditions and operating conditions and attempted to develop a vancomycin sensor showing high sensitivity even in whole blood. In this study, we investigated the polymerization conditions and attempted to develop a vancomycin sensor showing high sensitivity even in whole blood. The results are shown in Fig. 1. MIP electrode indicates same sensitivity in the whole blood and the buffer solution. However, a large background current was detected in the whole blood sample, which is probably due to coexisting redox species in blood. Thus, we are trying to study the mechanism of the background-current generation and to develop the correction-method of the current.
Introduction
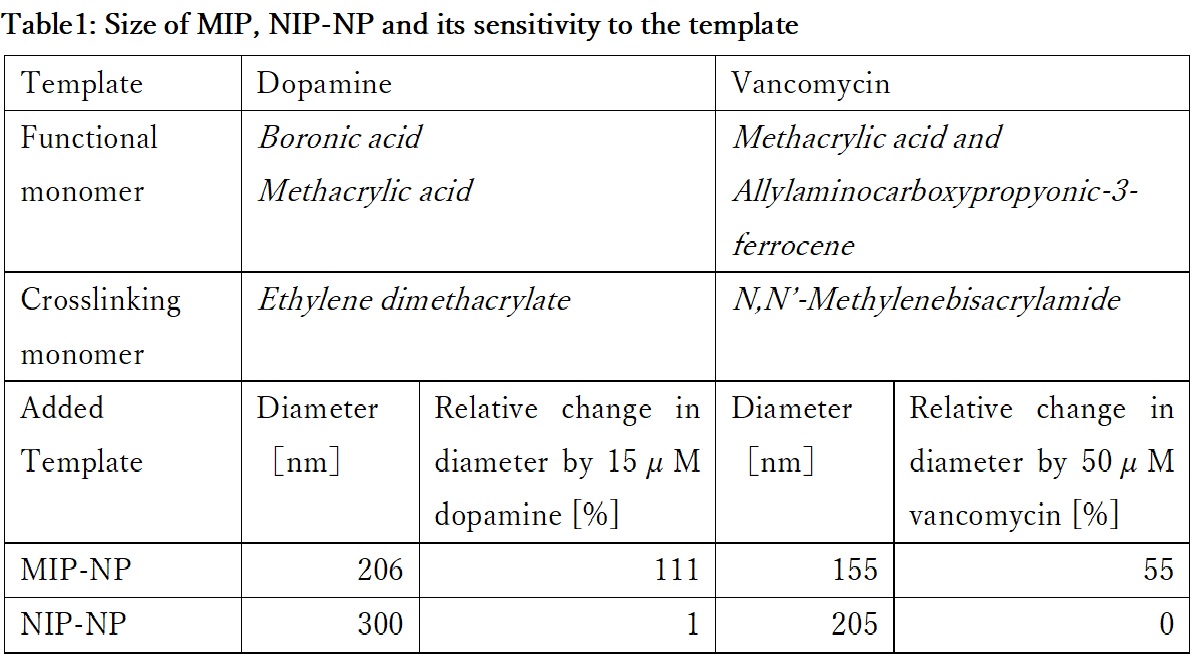
A molecularly imprinted polymer (MIP) is a polymer synthesized by molecular imprinting and characterized by binding specifically to a template substance. We are developing sensors using MIPs whose porosity is sensitive to the template specifically. In order to elucidate the mechanism of the sensor, we prepared MIP nanoparticles (MIP-NP) with the feature of increasing the particle size with respect to the template concentration. However presently, the mechanism by which MIP-NP increases the particle size is not known. Therefore, this time, MIP-NP is produced using various templates to elucidate the mechanism of MIP-NP particle size increase.
Experimental Methods
Dopamine or vancomycin were immobilized covalently on the aldehyde introduced surface of glass beads as a template. MIP was synthesized at the surface of glass beads by radical copolymerization of functional monomers and crosslinker shown in Table 1. MIP-NP was obtained by separating the MIP from glass beads surface. Non-imprinted polymer (NIP)-NP was prepared as the reference by the same operation except for omitting the template immobilization on the aldehyde introduced surface of glass beads. The particle diameter was measured by dynamic light scattering (DLS) spectroscopy.
Result and Discussion
The diameter of the particles are shown in Table 1. Each MIP-NP was smaller than respective NIP-NP. And MIP was expanded by the addition of the template, while NIP-NP was insensitive. MIP-NP possesses cavities imprinted by the template in the matrix, while the NIP does not. Those results indicate that the MIP-NP shrinks during the liberation from the template-immobilized surface and expands by rebinding with the template. Analysis of the relation between the size of MIP and the specific interaction with the template would be helpful for design of highly sensitive using MIP.
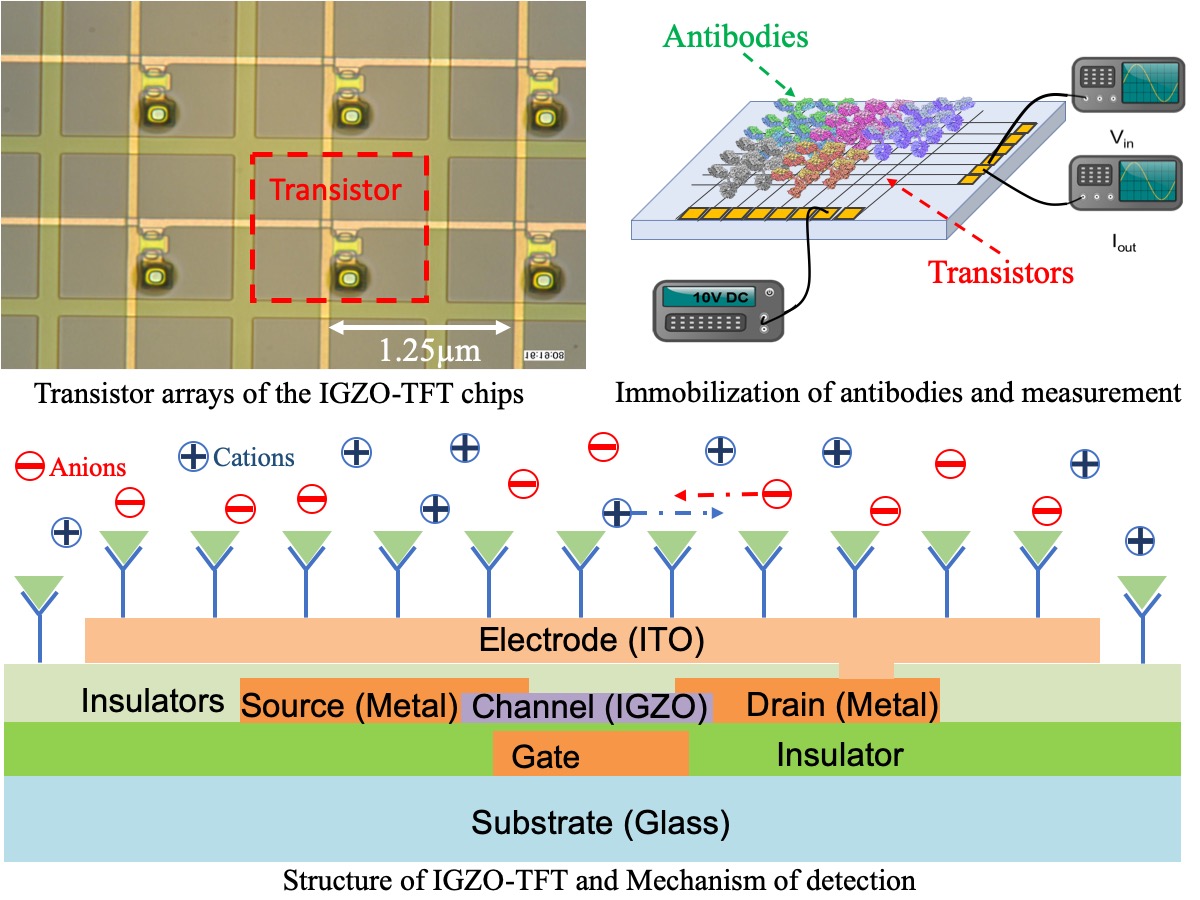
Thin film transistor (TFT) technology has been widely utilized in display, but seldomly been used in biosensing in spite of its priority in relatively high electron-mobility, economic-friendly manufacturing and lower energy consumption. We detected human serum albumin molecules by immobilizing anti-human albumin on dual-ligand modified surface of IGZO (indium gallium zinc oxide)-TFT chips with 400 transistors densely arrayed. AC power was applied on the chips and two neighboring transistors were activated. After recognition and combination, charged antibody-antigen pairs hindered movement of ions in medium between the two transistors due to electrostatic interactions, resulting in the change of permittivity of the dielectric and capacitance. By calculating output current, we could determine the impedance value, which would be reciprocally influenced by capacitance. We have detected the antibody-antigen reaction at the low level of 20 ng/ml for albumin molecules. Furthermore, we have compared 1 × PBS(Phosphate Buffer Solution), 0.1 × PBS(PBS : water = 1:9), 0.01 × PBS(PBS : water = 1:99) and 0.001 × PBS(PBS : water = 1:999) as solvents, where the results show that higher diluted PBS provides larger magnitude of signals for antibody-antigen reactions probably due to lower ion strength. The present IGZO-TFT biosensors is promising for a label-free multiple immunoassays method in the future. In next step, concentration of antigen will be decreased to determine sensitivity of the system and multiple types of antibody-antigen pairs will be immobilized and detected.
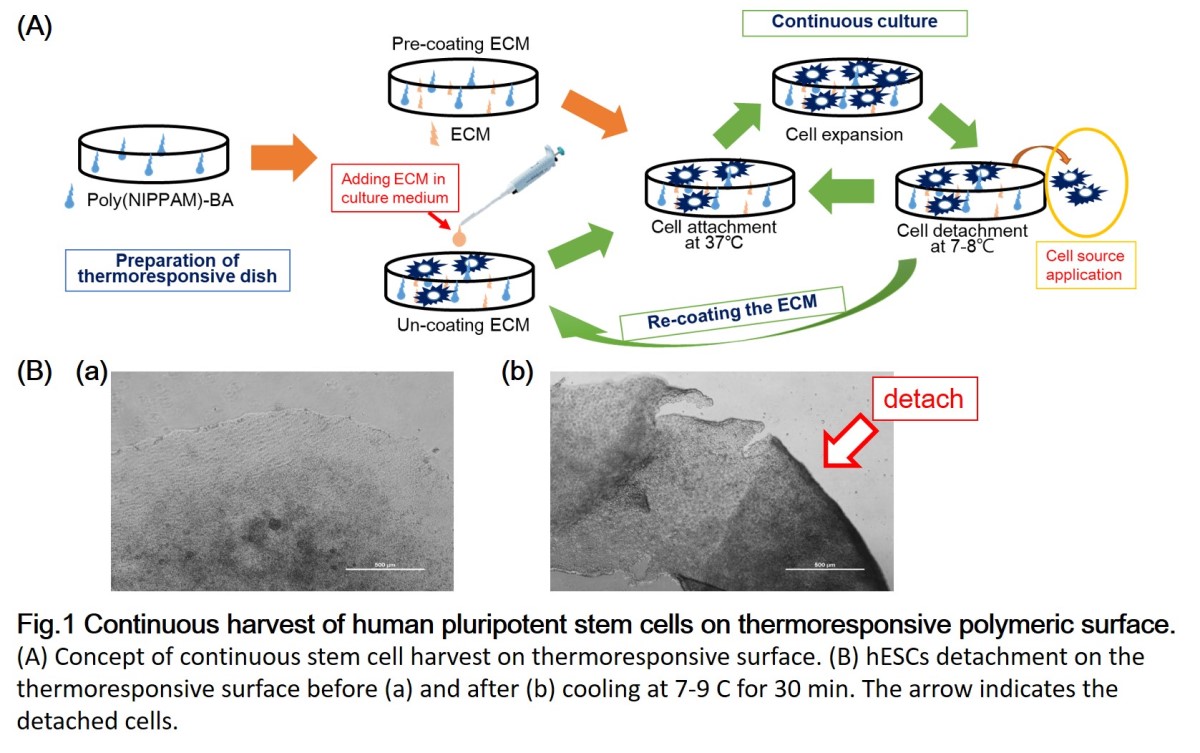
Human mesenchymal stem cells (hMSCs) are multipotent stem cells that can differentiate into mesoderm lineages such as osteocytes, adipocytes, and chondrocytes as well ectoderm (neurocytes) and endoderm lineages (hepatocytes) without generating tumors. The advantages of hMSCs used in cell therapy are their stemness, easy isolation, and no ethical concerns. These make them to serve an ideal candidate for regenerative medicine. However, the differentiated cells should be typically purified by magnetic-activated cell sorting (MACS) or fluorescence-activated cell sorting (FACS). This is laborious and expensive process. In this study, we developed a cell sorting dishes using thermoresponsive polymeric surface for isolation of targeted differentiated cells. hMSCs were differentiated into desired cell lines on the thermoresponsive surface and subsequently the temperature in culture medium was reduced below lower critical solution temperature (LCST), which enables to separate differentiated cells from non-differentiated cells. This method is an easy and economical process to be able to apply into mass production in future. Therefore, the sorting process on thermoresponsive polymeric surface should be crucial for future regenerative medicine application.
In this study, we first developed the establishment method of primary human amniotic fluid stem cells (hAFSCs) and human adipose stem cells (hADSCs) under the xeno-free condition. Subsequently, we designed the culture method of hAFSCs and hADSCs on thermoresponsive polymeric surface coated with poly(N-isopropylacrylamide), PNIPAAm, and several ECMs, such as (1) collagen I (2) fibronectin (3) synthemax II (oligo-vitronectin based substrate) (4) rVN (recombinant-vitronectin). After differentiation of hAFSCs and hADSCs into osteoblasts, we reduced the temperature in culture medium below LCST and detached the cells on the thermoresponsive surface (Fig. 1). We will investigate optimal PNIPAAm-ECM combinations for the differentiation of hAFSCs and hADSCs as well as the effect of cell sorting characteristics, which should be useful for the application in the regenerative medicine.
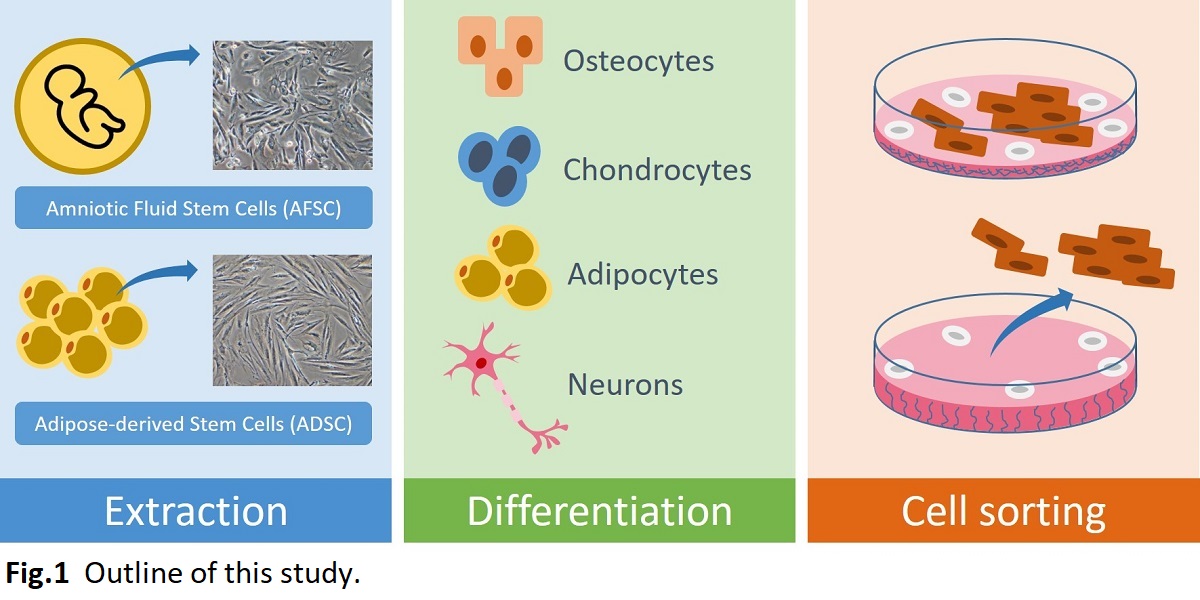
Microporous monoliths have been attracting increasing attention as separation materials due to their structural features. Poly(methyl methacrylate) (PMMA) is a polymer having biocompatibility and is insoluble in lower alcohols while it is soluble in the alcohols containing a small amount of water. Thus, microporous monoliths of PMMA can be made from its solutions in lower alcohols containing water via thermally induced phase separation method. In this study, we prepared composite monoliths of PMMA containing particles of hydroxyapatite (HA), which is one of the biocompatible inorganic materials that adsorbs proteins. The adsorption characteristics of proteins on the monoliths were also evaluated.
PMMA was dissolved in ethanol containing 20% water and HA powders at 70 °C. The polymer solution was then cooled for 1 h in a stainless-steel cup. After cooling, the solidified polymer was taken out and immersed in water to prepare a monolith. Bovine γ-globulin (BGG) and bovine serum albumin (BSA) in sodium phosphate buffer were adsorbed on a disk-shaped monolith in a custom-made holder and desorbed by raising the buffer concentration. The protein concentrations were determined by the bicinchoninic acid method and electrophoresis was performed to examine their adsorption characteristics.
The composite monoliths were successfully prepared by cooling the polymer solution at 0 °C. The dispersion of HA particles was improved by the pretreatment of HA powders with an aqueous solution of a nonionic surfactant Tween 80 before suspending in ethanol. The prepared monolith adsorbs much BGG and slightly BSA in 20 mM phosphate buffer (pH 6.8). The adsorbed BGG on the monolith was eluted with 400 mM phosphate buffer. The electrophoresis showed that BGG was separated from the mixture of BGG and BSA. We also examined adsorption characteristics of protein conjugates on the monolith. This study was partly supported by Kakenhi and Manufacturing Technology Association of Biologics, Japan.
For the improvement and change of the function of protein, the amino acid residues in the protein are selected and they are mutated to appropriate amino acid residues. Error-prone PCR is one of common method to improve and change the function of proteins; however, the identified variant is not always the one with enough function: In the error-prone PCR approach, the residue positions which should be mutated might be identified, but the selected amino acid at each position is not the most appropriate residue at each position. In this study, we combined the saturation mutagenesis with error-prone PCR: positive candidate amino acids at the critical positions identified by error-prone PCR were decided by means of saturation mutagenesis, and a smart-sized synthetic library was prepared to discover the variants with most appropriate function.
Here, we applied this methodology for improve the affinity of anti-human podoplanin antibody, LPMab-16, for rat podoplanin without deactivation against human target. The single chain fragment of variable region (scFv) was prepared from the sequences of humanized LPMab-16 Fv. For improvement of the affinity for rat podoplanin, w the scFv were mutated by means of error-prone PCR positive clones were selected from the variant library by means of phage display method. In the comparison of primary structure between positive and wild-type clones, the positions which should be mutated to promote affinity were determined.
In the secondary step, saturation mutagenesis was independently conducted at each decided position and the affinity of all the variants for both human and rat podoplanins were analyzed to determine amino acid candidates. Small-sized library was generated by conducting simultaneous saturation mutagenesis with the decided amino acid candidates, and the positive clone with 27 times higher affinity for rat podoplanin was able to be identified without deactivation for the binding to human podoplanin.
Polyacrylonitrile (PAN) nanofiber membrane was prepared by electrospinning technique. The PAN membrane used in this work comprises a polyethylene terephthalate (PET) spunbond fabric as a supporting layer with upper and lower PAN nanofiber membrane. After 3 M NaOH and diluted HCl treatments, the weak cationic exchange membrane (i.e., P-COOH) was obtained. The P-COOH membrane was then functionalized with tris(hydroxymethyl)aminomethane as an affinity nanofiber membrane (i.e., P-Tris). In this study, lysozyme was chosen as a model protein. The physical properties of the nanofiber membranes were characterized in terms of fiber diameter, porosity and pore size, specific area of the surface, FTIR and SEM analysis. The adsorption experiments were carried out in a well-mixed system under the various operating conditions (e.g., modification pH, adsorption pH, and the molar ratio of reactants, P-COOH/Tris). The dynamic adsorption characteristics of the P-Tris membranes for lysozyme by membrane chromatography were assessed by measurements of the breakthrough curves. The influences of operating conditions (e.g., adsorption pH, lysozyme concentration, no. of sheet membrane, and flow rate) on the adsorption performance of membrane were investigated in an AKTA prime chromatographic system (GE Healthcare).
Lipid modification of proteins regulates important biological events because the attached lipid moiety activates protein function by affinity to cellular membrane. The lipidated proteins also work for anchoring on drug delivery carriers composed of a lipid bilayer such as liposome. Therefore, protein-lipid conjugate is an effective way to functionalize proteins, and the strategy has attracted attention of researchers in various fields. Herein we focused on cross-linking reaction between specific glutamine (Q) and lysine (K) residues catalyzed by microbial transglutaminase (MTG) to achieve protein-lipid conjugate. Since MTG shows strict substrate recognition and site specificity under mild conditions, it has been demonstrated an effective biocatalyst for labeling proteins with artificial lipid-fused peptide substrates [1, 2]. A recombinant enhanced green fluorescent protein (EGFP) fused with amino acid sequence containing MTG-reactive Q (LLQG) at the C-terminus (abbreviated as EGFP-Q) was expressed in E. Coli. As for K substrate, lipid-fused peptides with MTG-reactive K (lipid-G3S-MRHKGS: abbreviated as lipid-K) were synthesized by Fmoc solid phase synthesis. Prepared EGFP-Q and lipid-K were cross-linked by MTG in homogenous water-phase, and obtained EGFP-lipid after MTG reaction was purified in an established method [2]. Finally, mixing EGFP-lipid and liposomes possessing various charges led to the decoration of liposomes with EGFP-lipid, especially the combination of EGFP-lipid and cationic liposome showed the enhancement of the anchoring ability on cellular membrane.
[1] M. Takahara et al., ACS Appl. Bio Mater., 1, 1823-1829 (2018).
[2] M. Takahara et al., Chem. Eur. J., accepted (2019).
Acinetobacter sp. Tol 5 shows high adhesiveness to various abiotic surfaces from hydrophobic plastics to hydrophilic glass, and stainless steel. This adhesive property is mediated by fiber protein AtaA, which is a member of the trimeric autotransporter adhesin (TAA) family. The adhesive feature of AtaA can be conferred to other non-adhesive Gram-negative bacteria by transformation with ataA gene, and bacterial cells displaying AtaA on their surface can be quickly immobilized onto various types of supports and used as immobilized whole-cell catalysts. While it has been reported that the adhesiveness of Tol 5 through AtaA is nonspecific, material preference of Tol 5 has never been evaluated quantitatively. In this study, we compared the adhesiveness of Tol 5 among various material surfaces. Materials used in this study was polystyrene, glass, stainless steel, and anti-adhesive materials, such as polytetrafluoroethylene (PTFE), Mica, poly (oligo (ethylene glycol) methacrylate) (pOEGMA) brush, and 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer. Bartonella henselae and Yersinia enterocolitica, which adhere to abiotic surfaces through their TAAs, were used as control bacteria. The bacterial cell suspension was applied onto material surfaces. After a certain time incubation, adhered cells were stained with crystal violet and quantified by measurement of the absorbance at 590 nm. Although Tol 5 showed high adhesiveness to most of the materials, the amount of the adhered cells depended on the materials. This result suggests that Tol 5 has material preference for cell adhesion. These findings give us a clue to elucidate the mechanism of the nonspecific, high adhesiveness of AtaA.
Solid-fat microspheres (SFMSs) are micrometer-sized spherical particles consisting of solid fat having a high melting point. They can be utilized for oral delivery of lipophilic bioactive components as well as preparation of powder fats. Generally, SFMSs are produced by solidification of oil droplets in oil-in-water (O/W) emulsions containing solid fats liquefied at high temperature. However, formation of fat crystals on the surface of oil droplets during their cooling process often cause flocculation and partial coalescence, and it results in instability of SFMSs. Therefore, stabilization of SFMSs during their formulation and storage processes is needed to improve food quality and shelf life.
In this study, we tried to stabilize SFMSs by coating with protein and polysaccharide using the electrostatic deposition technique. Monodisperse O/W emulsions containing palm oil droplets as starting materials of SFMSs were prepared by the microchannel (MC) emulsification technique. MC emulsification is a method for preparing highly monodisperse emulsion thus it is useful for finding out the effectiveness of biopolymer coatings on the stability of emulsions and SFMSs. MC emulsification was carried out using silicon MC plates installed to MC module at 65 °C. Sodium caseinate (SC) solution was used as the continuous phase and palm oil was used as the to-be-dispersed phase. Monodisperse O/W emulsions with mean droplet diameters of a few ten micrometers and coefficient of variation lower than 5% were successfully obtained. SFMS dispersions prepared by cooling the O/W emulsion were mixed with chitosan (CHI) solution to obtain SFMSs coated by CHI/SC layer via electrostatic interaction between positively charged CHI and negatively charged SC (Fig. 1). The CHI/SC ratio affected the diameter distribution and stability of SFMSs. During long-term storage at room temperature and heating-cooling treatment process of SFMSs, formation CHI/SC layer showed remarkable stabilizing effect for SFMSs by suppressing “oiling off” and fat crystallization.
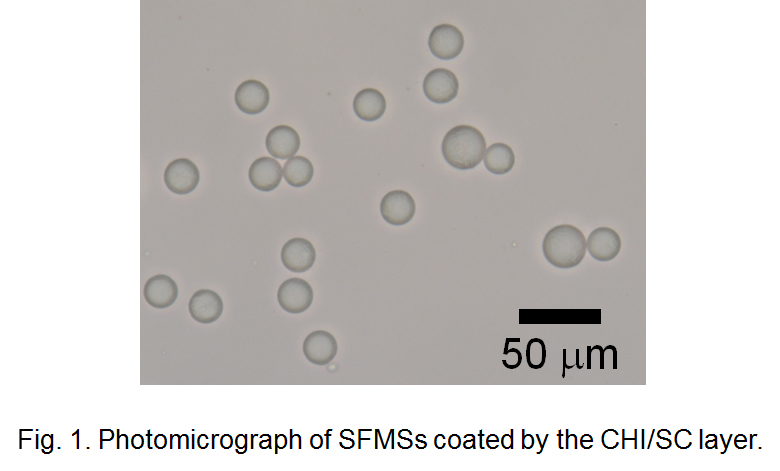
Liquid marble (LM), a self-standing microdroplet covered with hydrophobic polymeric particles, forms discrete space that can control physical and chemical exchange of substances between inner and outer environment. LM has been used as a micro-(bio)reactor because it provides easy-to-handle compartment for biological entities. In this study, we studied cell-free protein synthesis (CFPS) in LM, and the simultaneous hydrogelation of internal aqueous phase was tested to stabilize the whole system. Hereafter, we designated the LM in which the inner aqueous phase is a hydrogel state as hydrogel marble (HM). First, we constructed an LM/HM system by encapsulating the aqueous solution of CFPS components and the hydrogel precursors in LM. It was confirmed that CFPS in LM is possible under appropriate conditions (high humidity and suitable temperature) and enzymatic hydrogelation also proceeds to yield HM. As a result, capturing and immobilizing the cell-free synthesized proteins in hydrogel network was achieved, and by altering the hydrogel components and introducing cysteine residue to the target recombinant protein leaded to the efficient protein immobilization in HM [1]. We then tried to detect the gene after taking out from HM to apply the present system to directed molecular evolution. First, we prepared HMs containing the blank or target plasmid DNA encoding the gene of a target enzyme. Eventually, HMs exhibiting enzymatic activity are visually discriminated by floating HM on a substrate solution. HMs were taken out randomly, and the plasmid DNA coding target enzyme was detected only from the colored HMs. These results suggest the potential of HMs to utilize as a reactor to match genotype in LMs and phenotype in HMs. Quantitative evaluation of enzymatic activity produced by CFPS would be the next target for point-of-care testing and diagnostics.
[1] N. Kamiya, et al., Biotechnol. J., 13, 1800085 (2018).
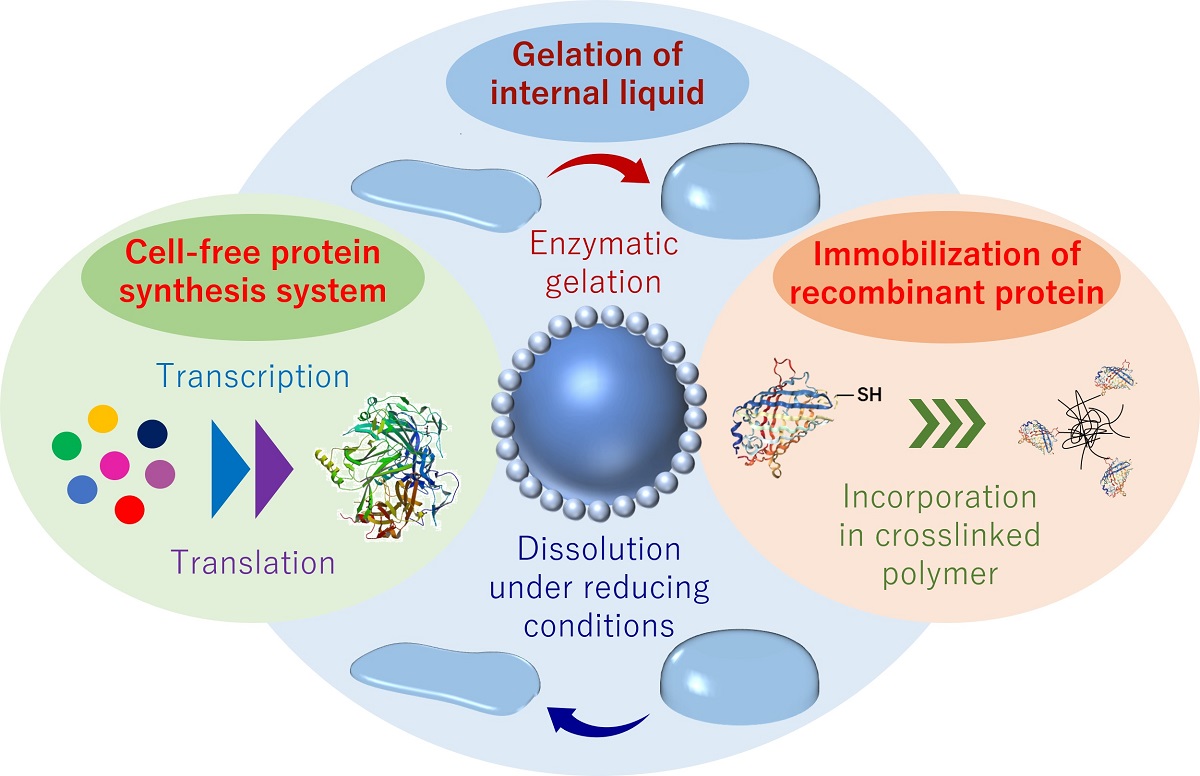
Analysis of the secretion of neurotransmitters in the nervous system is important for elucidating the mechanism of neural network. Then, development of a probe which can track a specified neurotransmitter in real time with high selectivity has been required. Molecularly imprinted polymer (MIP), which is a molecular recognition material obtained by polymerization with a template-effect of the target molecule may be applicable for the probe. In this study, nanoparticle of MIP including fluorescent group (fMIP-NP) were developed as the probe of dopamine secration. The serotonin, as the template, was immobilized on glass beads by using mixed anchor (1:1 in molar ratio) of 3-aminopropyltrimethoxysilane and 3-(2-aminoethylamino) propyltrimethoxysilane with glutaraldehyde. The template-immobilized beads were fluidized in a mixed solution of a fluorescent monomer, a template-affinity monomer, and a crosslinking monomer under UV irradiation. The colloidal fMIP-NP was collected from the surface of the beads by washing with N,N-dimethylformamide. And the dispersion medium of the colloidal fMIP-NP was replaced with 0.15 M phosphate buffer. The fluorescent intensity of the fMIP-NP was increased by addition of dopamine but was insensitive to L-3,4-dihydroxyphenylalanine (L-DOPA) as shown in Fig. The fluorescent nanoparticle prepared without the template was insensitive to both of dopamine and L-DOPA. Those results indicate that the specific interaction between the dopamine and the dopamine-imprinted cavity in the enhances the fluorescent intensity of fMIP-NP. The in vivo concentration of dopamine is said to be several nM. The relative change in the fluorescent intensity of the fMIP-NP is 4.2 % to the dopamine concentration of 15 nM which corresponds to the concentration of dopamine in vivo. Thus, the dopamine secretion in nervous system can be detected by diversion of a fluorescent voltage sensitive dye imaging system (e.g. MiCAM series in Brain Vision Inc.), which can detect relative change of 0.05% in fluorescent intensity.
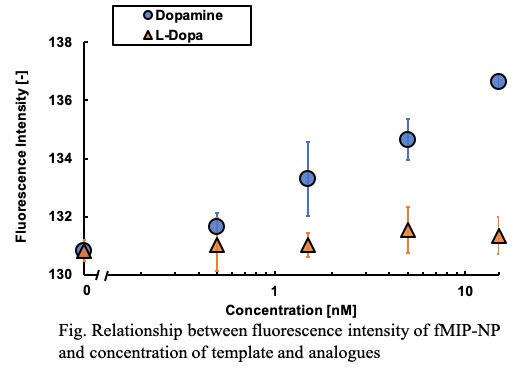
Molecular evolution based on mutagenesis is widely used in protein engineering. Recent advances in genetic engineering allowed us to prepare an extremely large library, beyond the limit of organic synthesis. However, such a large library are often difficult to obtain optimal proteins due to a large sequence space and finally leads to high costs in screening experiments. The success of protein engineering crucially depends on preparing a small library with high enrichment of functional proteins. Here, we propose a novel approach that combines molecular evolution with machine learning. In this approach, we conduct two rounds of mutagenesis where an initial library of protein variants is used to train a machine learning model to guide mutagenesis for the second round library. This enables us to prepare a small library suited for screening experiments with high enrichment of functional proteins. We demonstrated a proof of concept of our approach by altering the reference green fluorescent protein so that its fluorescence is changed into yellow. A small library of GFP variants was generated by means of point saturation and site directed random mutagenesis. Sequence and functional data acquired from the variants in the library were used for training a machine learning model to create the second round library. Mix primer techniques in overlap extension polymerase chain reaction generated the second-round library which consisted of 80 variants proposed by machine learning. The library turned out to contain yellow fluorescent proteins in high number. We successfully obtained a number of proteins showing yellow fluorescence, 12 of which had longer wavelengths than the reference yellow fluorescent protein. These results show the potential of our approach as a powerful method for directed evolution of fluorescent proteins.
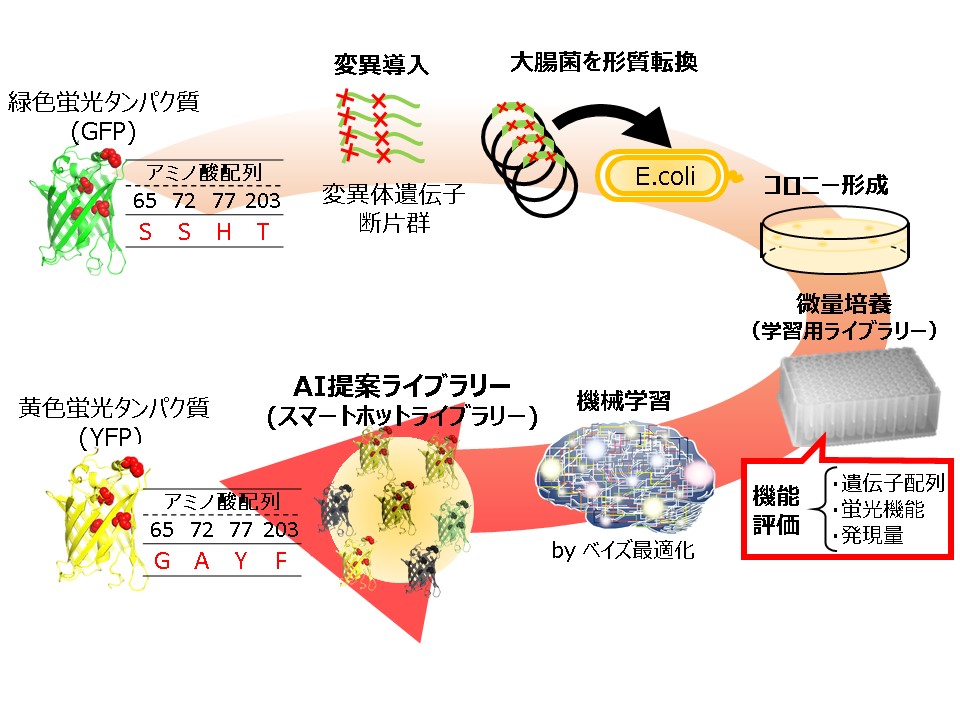
The adsorption of lysozyme on Reactive Gren 19 dye–ligand affinity nanofiber membrane was investigated at 25 °C in a batch system. The effects of operating parameters such as initial pH, immobilization dye concentration, and initial lysozyme concentration on the adsorption were analyzed using Taguchi method. The optimum adsorption conditions were determined as initial pH 7, immobilization dye concentration 3 mg/ml, and initial lysozyme concentration 3.0 mg/ml. At optimum conditions, the adsorption capacity of dye–ligand affinity membrane for lysozyme was found to be 686 mg/g. Based on the optimal conditions, the thermodynamic and kinetic studies were investigated under various operating conditions, such as adsorption pH, lysozyme concentration and temperature. To purify the lysozyme from chicken egg white by using the membrane chromatography, it was to find the optimal purification strategy, including adsorption (e.g., adsorption pH, concentration of CEW, and operating velocity), wash (e.g., liquid velocity) and elution (e.g., type of salt and concentration and liquid velocity) stages. Under the optimal conditions, the results showed that the lysozyme was recovered with a high yield of 98.5% and a high purification factor of 143.3 in a single step.
S-Adenosylmethionine (SAM) is an important methyl donor involved in the methylation of diverse biomolecules, including DNA, amino acids, and secondary metabolites. Although SAM-dependent methyltransferases can become versatile tools for the biocatalytic manufacturing of various useful compounds, SAM-dependent methylation has not yet been industrialized due to the complexity in SAM regeneration. In living cells, SAM is demethylated to S-adenosylhomocysteine (SAH) in association with the methylation of recipient substrates, and subsequently degraded to homocysteine, adenine, and ribose. Homocysteine is then re-methylated to methionine by using another methyl-donor molecule, methyl tetrahydrofolate (THF). Finally, SAM is regenerated by adenylation of methionine with ATP. Although methyl THF can be produced from serine or glycine, carbon sources taken up in the cells are mostly routed into energy production and other biosynthetic pathways, limiting the amount of carbon input which can be utilized for the production of methyl THF and following SAM regeneration.
In this study, we newly designed a SAM-regeneration pathway, in which methanol is used as a source of methyl group, and installed it into Escherichia coli. A heterologously expressed methanol dehydrogenase (MDH) catalyzes NAD+-dependent oxidation of methanol to formaldehyde, which is then spontaneously conjugated with THF to yield methylene THF. An endogenous enzyme in E. coli (methylene THF dehydrogenase) mediates NADH-dependent reduction of methylene THF to methyl THF, which serves as a methyl donor to regenerate methionine from homocysteine. We demonstrated that integration of an methyltransferase into the recombinant E. coli equipped with this pathway allowed methylation of a recipient substrate into its methylated derivative using methanol as a source of methyl group. Moreover, deregulation of SAM biosynthesys and regeneration further enhanced methylation reaction.
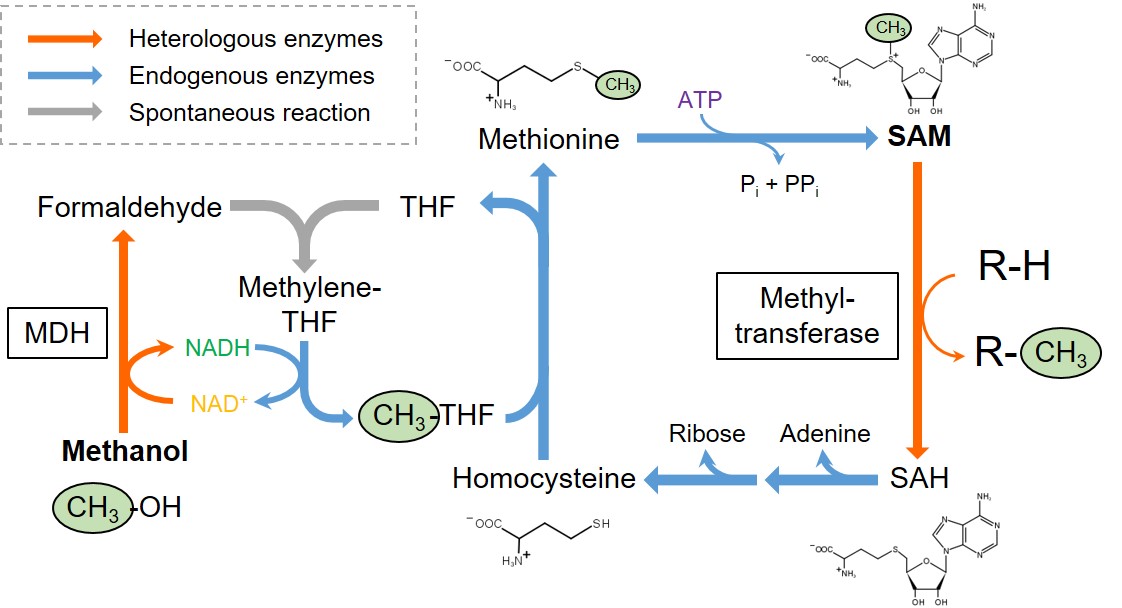
Lipase catalyzes ester hydrolysis under an ordinary temperatures and pressures. In organic solvent, lipase catalyzes ester synthesis and transesterification and lipase has substrate specificity. Lipase is one of important enzyme for industrial application. However, lipase is immediately inactivated in the presence of organic solvent in which the substrate dissolves. Enzyme immobilization is one of solution to improve the stability of enzyme. Yasuda synthesized the amphiphilic macromonomer (2-[p-(1,1,3,3-tetramethylbutyl) phenoxypolyethoxy] ethyl methacrylate, MAX-n, n is the degree of polymerization of the polyethoxy group). Amphiphilic particles obtained by seed copolymerization of MAX-n was a good immobilization support of lipase. In hexane, immobilized lipase on amphiphilic polymer particles had long-term activity and good transesterification activity. However, low immobilization amount of lipase was a problem. In this study, to enhance immobilization amount of lipase, we synthesized amphiphilic polymer micelle of MAX-n and polymer micelle was incorporated into pores of porous particle. The degree of polymerization of the polyethoxy group of n was affected immobilization amount of lipase and stability of lipase in the presence of hexane. When the degree of polymerization of the polyethoxy group was 4.39, the half-life of lipase in hexane was extremely long at 8500 hours.
Krill oil contains huge amount of long chain polyunsaturated fatty acids (PUFAs) such as eicosapentanoic acid (EPA) and docosahexanoic acid (DHA). However, the easily oxidizable characteristics of DHA and EPA make a short shelf life of those compounds. To increase the oxidative stability of EPA and DHA, krill oil was encapsulated in Saccharomyces cerevisiae yeast cells. Rosemary oil (RO), an antioxidant, was added with an amount of 5 wt% of krill oil to reduce the degradation rate of EPA and DHA. The solid content of feed solution was 25 wt%, where the ratio of yeast to krill oil was 2:1. The slurry of yeast, krill oil and water were incubated at 40 °C for 4 h. Non-encapsulated oil was removed by centrifugation and washed with distilled water before spray drying to reduce the surface oil in spray-dried powder. A pilot scale spray dryer (Ohkawara L-8 type) was used to encapsulate krill oil in yeast cells. Four different powders were prepared, where RO was added in two powders and another two powders were without RO. One powder without RO and one powder with RO were washed by hexane after spray drying. Around 0.5 g powders were put in the small vial and placed the powders in drying oven at 50 °C. Thin layer chromatograph with flame ionized detector (TLC-FID) was used to analyze total oil and surface oil content of encapsulated powders. The maximum total oil and surface oil was found around 0.058 g/g-yeast powder and 0.01 g/g-yeast powder, respectively in the powder with RO and hexane washing. The content of EPA and DHA in the powder with RO had higher value than that of powder without RO after the storage of 4 weeks at 50 °C storage.
There are two methods of filleting process of large size frozen fish in the early stages of distribution : a method of cutting thawed fish with a knife and a method of cutting frozen fish with a band saw. The method of cutting thawed fish with the knife has problems in terms of hygiene and freshness. On the other hand, the method of cutting frozen fish with the band saw has problems in terms of safety and yield. We have proposed “cryo-cutting” as a cutting process for frozen fish without using the band saw. Samples were prepared as follows ; a thawed bonito (whole fish body) was cut into a cylindrical shape by knife and re-frozen at a temperature range from -70°C to -40°C. Notched samples was prepared by cutting a V-shaped notch on the back side of the fish sample along the dorsal fin. To try to fillet bonito, a cylinder splitting load was applied to the samples with and without the notch. A wedge-shaped jig was attached to the upper compression plate and pressed into the back side of the sample or into the notch of the samples. As a result, the sample could be split into two parts along the median plane of fish body. By using the jig, it was possible to certainly split into two parts in processing temperature range from -60°C to -40°C. Furthermore, in this temperature range, the load required for splitting could be significantly reduced by 50% and more. The reduction in stress was due to stress concentration at the notch tip. These results have shown the possibility of increasing the cutting accuracy and reducing the cutting stress, by use of the wedge-shaped jig. This study could be a new method for filleting frozen fish.
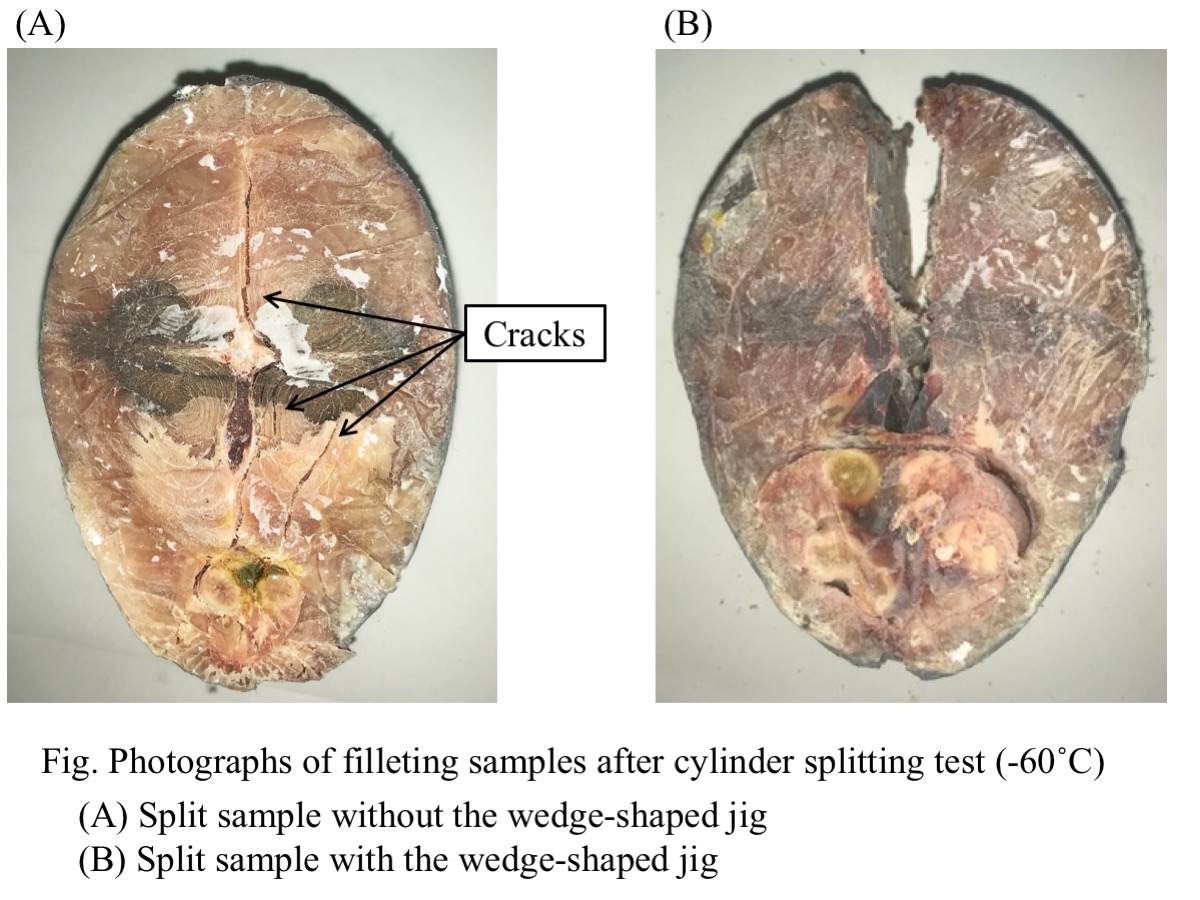
Peptides are heteropolymers composed of amino acid residues linked by peptidic bonds between the carboxyl group of one amino acid residue and the α-amino group of another. They are molecules of paramount importance in several fields, especially in hamaceuticals, health care and nutrition fields.
Peptides are mainly obtained from solid phase peptide synthesis (SPPS) as an established technique. SPPS is a cyclic process with four steps in each cycle: deprotection, washing, coupling, and rewashing. As a result, SPPS is typically performed with long coupling time, large excesses of reagents, and many repetitive washing steps between each steps.
Microfluidic reactors for biomolecule syntheses have many advantages over conventional bulk reactors. Benefiting from minimization of the reaction scale and enlargement of the specific surface area, the microfluidic chemical reactions result in improvement of yields, selectivity, safety and efficiency.
We report our development of flow peptide synthesis in a microchannel. The flow peptide synthesizer consisting of the microchannel with the width of 1mm and depth of 0.2mm on a glass plate and a filter for resin trap. During a coupling reaction, a syringe pump made reciprocating flow of resin beads suspension in the microchannel to restrain aggregation and blockage of resin beads.
Model peptides (Met-enkephalin) were synthesized in a continuous-flow manner. Coupling time of 10 min gave better yields and less side products than that of 40 min with a conventional batch synthesizer. Additionally, two equivalent of amino acid gave good yields similar to four equivalent of amino acid in the conventional batch synthesizer. Therefore, the flow peptide synthesis in a microchannel can contribute to significant reduction of the total coupling time and consumption of amino acid.
High density adsorbent STREAMLINE Direct HST (GE Healthcare) was used to investigate the fluidization and axial liquid mixing characteristics under various operating conditions (e.g., liquid velocity and viscosity and rotational speed) in a specially designed stirred fluidized bed column (SFBC). The results showed that the experimental Richardson-Zaki constant of n is approximately 3.6-4.7, and the experimental terminal velocity of the adsorbent particle is some degree different from the theoretical value (< 20%). Based on the liquid viscosity and density, and adsorbent density and particle diameter, the bed height in the fluidized bed at a desired liquid velocity can be estimated. More experiments were conducted with SFBC for investigating the axial liquid mixing using 5% acetone (v/v) as a tracer and the fluidized bed height was maintained at a constant bed height (i.e., two times settled bed height). The results revealed that the axial liquid mixing was much affected by the liquid velocity and viscosity, and the rotational speed of agitator. As the rotating speed was increased in a higher liquid viscosity, the liquid flow was found to be more stable. Hence, the rotational speed was significantly contributed to the liquid mixing efficiency.
The medical application of cell engineering has attracted a lot of attention. For example, circulating tumor cells (CTCs) separated from blood offer tremendous potential for diagnosis of cancer. The analysis of CTCs is would be useful for progressions of precision medication. In order to attain those medication, it is essential to develop the cell separation technology. Centrifugation, which is based on weight and density, and affinity separation are conventional cell separation method. In addition, cell size and deformability also play a role in cell separation. A novel cell separation method based on size and deformability is worth of development, because it enables the cell separation without rare protein and marker.
In this study, we applied the metal mesh device (MMD, Figure 1.) to the cell separation. MMDs are thin metal films with a through hole structure with precisely controlled pore size. The well defined pore structure of MMD would be appropriate to separate cell based on size and deformability. The aim of this study is achieving cell separation of MMD only with the physical property of cell, and obtaining basic information.
We will report the cell separation of several cell lines with MMD. Since HL60 (floating cell) and HeLa (adhesion cell) cells were reported to have different size and deformability, the cell separation of those cells were conducted with MMD. The permeability of MMDs with different hole size was studied. We carefully observed the cells having high and low permeability, and investigated the relationship between permeability and physical properties.
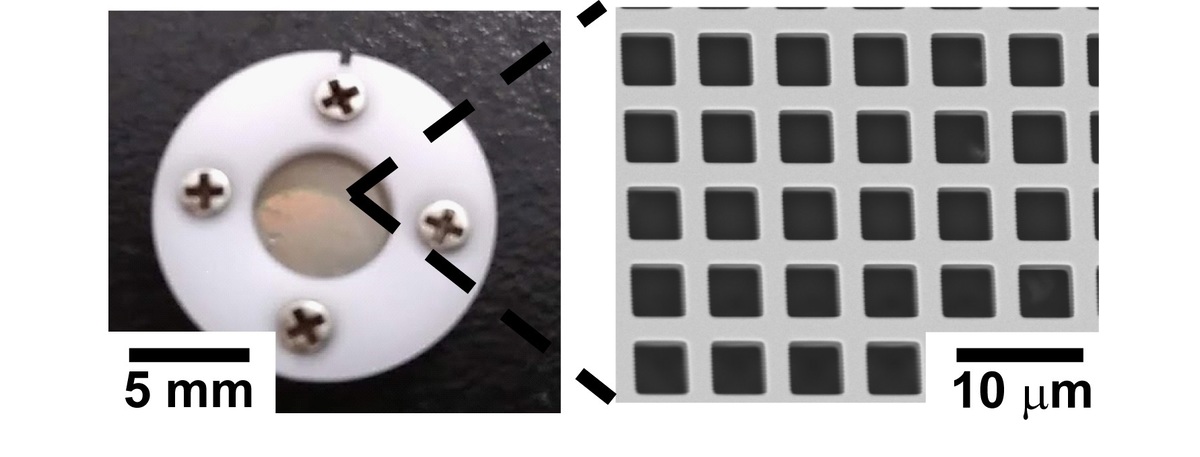
Efficient oxygen supply in bioprocesses is required for aerobic microbes, which demand oxygen as the final electron acceptor in the respiratory chain reaction. In general, compressed air as babbles through a sparger is introduced in a stirring tank reactor. The bubbles sizes are ~ mm of diameters, and stirring paddles collapses and disperses the babbles, and increases oxygen transfer rate. However, the stirring blades often damage the microbial cells, and requires large input powers. To solve the trade-off problem between efficient oxygen supply and cell damages, fine babble sparger (FBS) attempted to apply the microbial cultivation. Here, influence of FBS using on a biosurfactant-, mannosyletythritol lipids (MEL), production by an absolute aerobic fungi, Pseudozyma hubeiensis were investigated. Pseudozyma hubeiensis SY62 was inoculated in 1 L of a MEL-production medium prepared in a 2-L table top bioreactor equipped an alumina porous sparger with 1.3 μm diameter pores (FBS sparger, Noritake Co. Ltd.). Reference cultivation were performed in the equivalent condition except without the alumina porous sparger. Cell growths were measured by a dry cell weight method and the MEL concentrations were determined by a HPLC with an evaporative light scattering detector.
Agitating at 200 rpm, cell growth at the third day with FBS sparger increased to 19.2 g/l, which was higher than that without the sparger (5.7 g/l). Although MEL production were never observed without the sparger, the production reached to 4.7 g/l for three days. However, the significant differences in cell growths between with and without FBS sparger were not observed at 525 rpm. Interestingly, MEL productions for three days with the sparger were induced to 48.9 g/L, which was 1.7 fold larger than that without the sparger. The results indicates that aeration with SPG sparger can achives an efficient oxygen supply without excess stirring.
We have developed polymer particles (g-particle) having grafted polymer chain for adhesive cell culture. To keep adhesive cells on particles surface, a graft chain having both an epoxy group and a hydrophilic carboxyl group is required. This is because the reaction between the epoxy group and the amino group of the extracellular matrix facilitates cell adhesion. The cells grown on g-particle forms three-dimensional matrix in the particles gap and three-dimensional cell matrix has many physiological functions. However, when cell suspension added to g-particle dispersion, the rate of cell growth and adhesion were not high. This was because g-particle moved freely without being dense state in the cell culture dish. In this study, to form and keep a packed state of g-particle by magnetic force, we tried to synthesize a new magnetic g-particle containing paramagnetic Fe3O4 nanoparticles. In suspension polymerization, monomer and hydrophobic modified Fe3O4 nanoparticles were polymerized and the graft polymerization of methacrylic acid and glycidyl methacrylate was carried out. Carboxyl group and epoxy group were introduced as graft polymer chains into the base polymer particles by using pre-incorporated azo groups in polymer particles as an initiator. The number average particle size of magnetic g-particles was 103 μm and they contained 5 wt% of Fe3O4 nanoparticles. They could be gathered by applying an external magnetic force. In addition, the adhesion and growth rates of cells were compared using conventional g-particles and magnetic g-particles. As a result, it was found that cell culture in the presence of magnetic g-particles shows better results.
Blood is one of the most important biological samples. This is not only an analysis target for diagnosis and research but also widely used for treatment. For example, it includes blood transfusion and blood products. Blood contains various blood cells and components that constitute plasma. Separation of these components is one of the most basic operations for its diagnosis, analysis, and utilization.
Conventionally, centrifugation is a general method of blood separation. Centrifugation is based on the specific gravity difference in principle. This is a batch method, thus, processing large amounts of blood requires large devices. In addition, it is possible to separate only target blood cells by using an appropriate centrifugation solution, however, precise separation is difficult.
In this study, we tried that the blood cell separation using a Metal Mesh Device (MMD Fig. 1) with precisely controlled pore size and pore arrangement. The MMD has a nickel mesh structure manufactured by MEMS technology. Therefore, MMD is expected to be applied as a separation membrane, because; it has a large number of through holes accurately designed in the submicron order. It is already reported that the collection and classification of PM2.5 present in the atmosphere using MMD*.
MMD as a separation membrane has an advantageous of blood separation. That is, separation can be achieved using the simple phenomenon of passage/capture when blood is introduced. Blood cells are expected to be separated according to the size and hardness of blood cells by the mesh structure precisely designed on the submicron order. In this study, we reported the blood cell passage rate in the case of using MMD with various pore sizes and evaluated the blood cell passage control by surface PEG modification.
* H.Seto, et al., Chem. Lett., vol. 43, 4, 408-410 (2013)

Biopharmaceutical proteins such as therapeutic antibodies are usually produced by recombinant Chinese hamster ovary (CHO) cells. High producer cell lines are screened from transfected cells with random integration of target genes. Since transgene expression is susceptible to the surrounding environment of integrated genomic locus, producer cell lines should be selected from a large number of recombinant cells with heterogeneous transgene insertion. In contrast, targeted integration or site-specific integration into a characterized genomic locus enables more predictable transgene expression and less clonal variability, and hence stable production of target proteins can be expected. Genome editing technology based on programmable nucleases such as TALEN and CRISPR/Cas9 has recently emerged as a versatile tool for precise editing of target locus in the cell genome. Previously, we have demonstrated targeted knock-in of transgenes into the hypoxanthine phosphoribosyltransferase (hprt) locus of CHO cells using TALEN- and CRISPR-mediated precise integration into target chromosome (PITCh) systems [Sakuma et al., Int. J. Mol. Sci., 16, 23849-23866 (2015); Kawabe et al., J. Biosci. Bioeng., 125, 599-605 (2018)]. Here, we attempted to generate targeted knock-in CHO cells based on the homology-independent targeted integration (HITI) system. Transgene cassettes in donor vectors (pHITI/DsRed) were flanked by gRNA recognition sites to eliminate the vector backbone sequences after integration into the cell genome. To evaluate the targeted knock-in efficiency of transgene cassettes, CHO-K1 cells were co-transfected with donor vectors and respective Cas9/gRNA expression vectors. After seeding of transfectants, we counted numbers of colonies showing both drug resistance and DsRed expression. For the established clones, the targeted integration was confirmed by genomic PCR using specific primer sets for joint-regions. The transgene knock-in efficiency into the hprt locus using HITI system was 6.7-fold and 5.0-fold higher compared with those using homologous region (HR) and PITCh systems, respectively.
>Biologics are gaining importance in treatment of diseases, such as cancer, because they are safer alternatives to the conventional treatment. They are mostly produced in bacterial, yeast or mammalian cells. When there is a need for a correct protein folding and posttranslational modifications of biologics, mammalian cell lines are a go-to. Chinese hamster ovary (CHO) cell lines are the dominant mammalian cell lines in production of biologics. They do not propagate human viruses, can grow in suspension cultures, protein folding and modification is human-like and the production of the CHO cells is high.
>The demand for biologics grows. To meet the quantitative demands for the new biologics at sustainable cost, we must further increase the productivity of the CHO cells. Here, we explore the effects of cell cycle inhibitors aphidicolin and caffeine on productivity of CHO cells. We inserted an IgG1-expressing plasmid into a CHO cell lines, which were either treated with aphdicolin or caffeine during transfection, or were not treated in control samples. Aphidicolin treated cells had a 3.5-times higher productivity than control, while caffeine had no effect. While trying to identify the reason for the productivity increase in aphidicolin-treated cells, we noticed that inserted gene copy number or gene expression level remained the same between the aphidicolin treated and control cells. The inserted gene mainly in chromosome 2 in control samples, while in treated samples, the gene was found on many different chromosomes, suggesting that the location of the gene integration might influence the productivity. Because there was no change in gene expression levels between the cell lines, we hypothesize that the difference arises in the downstream steps of protein synthesis. Because the aphidicolin damages the DNA in vicinity of microRNA genes, we further hypothesize that the changes in microRNA expression might contribute to the higher productivity.
The past decade has seen a significant transition from static and passive material to dynamic, bio-based, and stimuli-responsive biomaterials in the cell-seeding technology. Recent advances towards such biologically active hydrogels have been directed to design biomaterials with a superior feature for higher-order cell culture than conventional 2D culture. One obstacle in the fabrication of biologically active hydrogels is to develop in situ crosslinking of gel precursors without impairing bioactive agents to mimic the extracellular matrix condition. Enzymatic crosslinking methods have been suggested as a potential way to introduce external functionality such as sol-gel or gel-sol transition to cell culture scaffolds. Herein, we propose to utilize the horseradish peroxidase-mediated cross-linking that can enable both redox-responsive hydrogel formation of tetra-thiolated PEG via disulfide linkage and incorporation of chemically thiolated materials in the hydrogel. Enzymatic fabrication of redox-responsive hydrogel using different types of gelatin revealed that chemically thiolated gelatin (Gela-SH) and heparin (Hepa-SH) can be co-incorporated into the polymeric network, yielding the dual-functionalized hydrogels. Hepa-SH with different degree of thiol modification was incorporated by considering its optimum binding ability with growth factors and a conflicting property such as the intrinsic anti-proliferative effect. Interestingly, the type of gelatin had a significant effect on the physical and biological aspects of hydrogels; alkaline-treated gelatin (Gela(B)-SH) exhibited superior performance over acid-treated gelatin (Gela(A)-SH) to generate the dual-functionality, cellular adhesiveness and binding ability of growth factor, originating from the two natural biopolymers. Eventually, the Gela(B)-SH/Hepa-SH dual-functionalized PEG-based hydrogel supported both cellular attachment and binding of basic fibroblast growth factor (bFGF) under cell culture conditions, which increased the proliferation (~38%) and phenotype transformation (~89%) of NIH3T3 cells cultured on the hydrogel. This hydrogel system strategy also facilitated faster fibroblast (NIH3T3) and endothelial cell (HUVECs) confluency, which makes it possible to fabricate 2D cellular sheets efficiently.
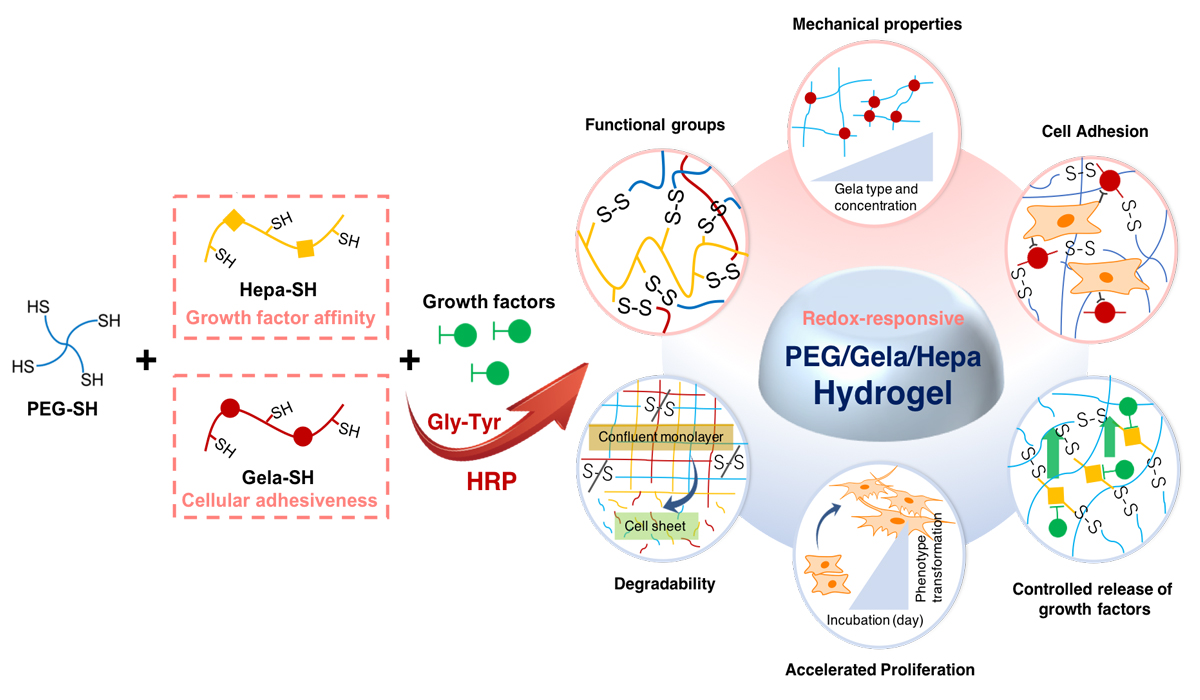
A carotenoid compound β-cryptoxanthin (BCX) is believed to have several functions for human health such as prevention of diabetes mellitus , carcinogenic inhibitory action and immunostimulatory action. However, bioavailability of BCX might be low when orally-administered, due to the extremely low solubility in aqueous media. Emulsification is one of the efficient techniques to obtain highly dispersible formulation for poorly water-soluble ingredients. In the present study, highly dispersible solid formulation containing BCX was prepared by emulsification techniques followed by lyophilization. Kumquat (Fortunella spp.) is a citrus fruit cultivated in Asian countries. BCX was extracted from dry kumquat using the mixture of pyrogallol and ethanol. An O/W emulsion was prepared using an ultrasonic homogenizer by mixing a hexane solution containing the kumquat extract, and an aqueous solution containing sodium caseinate and a sucrose fatty acid ester (L-1695) as an emulsifier. Subsequently, the resulting O/W emulsion was lyophilized to obtain a solid formulation. For the evaluation of the dispersibility, the formulation was added into an sodium phosphate-buffered solution. After filtration of the aqueous mixture using a φ0.80 μm membrane filter, concentration of BCX in the filtrate was determined using HPLC.
Under acidic and neutral conditions, the water-dispersibility of BCX for the solid formulation was much higher than that of kumquat extract alone. Addition of both L-1695 and sodium caseinate was effective to enhance the apparent solubility of BCX under wide pH conditions. Only in the presence sodium caseinate, the dispersibility was very small under weakly acidic condition which is close to isoelectric point for casein. Thus the solid formulation prepared by emulsification techniques was effective to enhance the dispersibility of BCX.
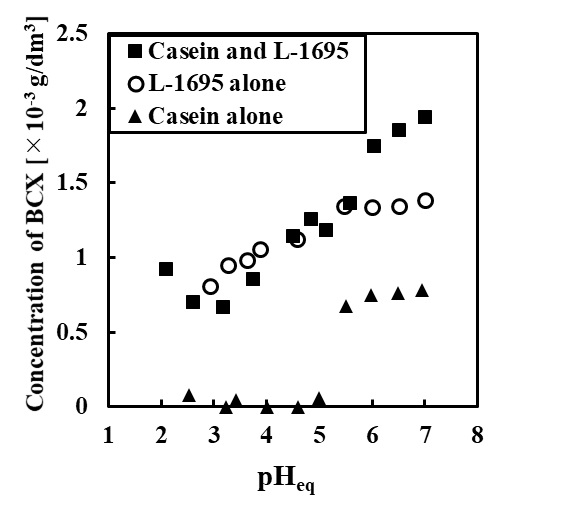
Hepatocytes are a promising cell source for liver tissue engineering and drug screening studies. For the success of such applications, hepatocytes have to maintain liver-specific functions at a high level. Various approaches, such as spheroid (three-dimensional cell aggregate) culture and co-culture, have been adopted to preserve hepatocyte functions in vitro. In this study, we constructed a co-cultured spheroid, which combined hepatocytes with other cells, and evaluated the effects of co-culture on the expression of hepatocyte functions.
As a culture platform for spheroid generation, we fabricated a microwell chip which contained 397 microwells (400 μm in diameter) on a poly-dimethylsiloxane substratum, and its surface was modified with 2-methacryloyloxyethyl phosphorylcholine co-polymer to create the non-adhesive area. In this study, three kinds of cells, primary rat hepatocytes (Hep), mouse fibroblasts (NIH3T3), and human adipose-derived stem cells (ADSC) were used for the spheroid culture, and the spheroid properties were compared in the Hep-spheroid, Hep/3T3-spheroid, and Hep/ADSC-spheroid conditions.
The formation of Hep-spheroid was required approximately 2 days of culture, but the spheroids formation was promoted with the presence of 3T3 cells or ADSCs. Although the 3T3 cells covered the spheroid surface, the ADSCs coagulated in the center of spheroid. Furthermore, almost same spheroid size was maintained in the co-culture conditions during culture period, but the size of Hep-spheroids decreased with the increase of culture time. The albumin secretion ability of co-cultured spheroids (Hep/3T3 or Hep/ADSC-spheroids) was higher than that of mono-cultured spheroid (Hep-spheroid), and especially the function of Hep/3T3-spheroids was highest under all conditions.
These results indicate that the co-cultured spheroids are a promising technique for the maintenance of hepatocyte functions, and the combination of cell species is important factor for modulation of cell functions.
Hydrogel materials have been widely used in tissue engineering and regenerative medicine research. For example, collagen and its derivatives are commonly used as scaffolds to incorporate cells and form 3D complex structures either by molding or 3D printing. However, these structures typically change their form dramatically in cell culture after fabrication due to cell traction forces and/or hydrogel degradation. Here, we propose a 4D (3D+time) tissue engineering approach that incorporates these changes in the design and completes the final structures during cell culture.
We encapsulated NIH 3T3 cells (RIKEN CELL BANK) in type I collagen (3 mg/mL, Nitta Gelatin Inc.) at different cell densities, and monitored the shape change for at least 3 days. Gelatin methacrylate (GelMA) was also used as a hydrogel to encapsulate cells. GelMA has emerged as a preferable hydrogel material in tissue engineering, especially for the applications of 3D bioprinting due to its photocrosslinkable and biocompatible features. By varying the UV crosslinking conditions (e.g. UV intensity and time) and GelMA concentration, as well as cell density, we observed different degrees of hydrogel shape changing during cell culture. We then fabricated bi-layer structures using two components with different shape changing properties. The bi-layer strips curved towards the side which had higher shrinking ratio (or lower swelling ratio). Their curling ratio was recorded and calculated. Furthermore, to demonstrate the capability of this 4D tissue engineering approach, we fabricated a bi-layer structure consisting 6 flower pedals, using collagen hydrogel with and without cells as the inner and outer layers, respectively. We observed the structure to form a flower shape by “closing” its pedals during the 3 days of culture.
In summary, our 4D tissue engineering approach can be employed to form designed structure after fabrication, and will be further explored for use of 3D bioprinting.
Chinese Hamster Ovary (CHO) cells are widely used as host cells in antibody production. The antibody production process has to aim towards high productivity and/or high titer, low operation costs and short production time. To improve productivity, cell flocculation can be applied in cell removal and/or cell culture processes to decrease impurity and turbidity. Calcium chloride is one of the cell flocculants that was studied and optimized for application [1,2]. In addition, low concentration calcium chloride is also being used as supplement in culture medium. In this study, we investigated that calcium chloride addition improved product titer, and decreased turbidity and impurities of cell culture supernatant after cell flocculation.
IgG-producing CHO-HcD6 cells were cultured as batch cultures using serum-free medium supplemented with calcium chloride at the beginning of the cultivation with 6 different concentrations (0.5, 1, 2, 5, 15 and 60 mM). Samples were recovered at the end of batch cultivation until cell viability dropped under 60 %. Cell and IgG1 concentrations during cultivation were analyzed using Vi-cell cell counter and ELISA, respectively. Dipotassium hydrogen phosphate was added at the end of cultivation, and the cells were removed from supernatants by centrifugation. Supernatants were collected for IgG1 concentration, turbidity (OD600) and host cell proteins measurements by CHO Host Cell Protein ELISA kit (Cygnus). Highest IgG1 concentration was 263 mg/L in 2 mM CaCl2. In addition, the turbidity of supernatant after centrifugation was decreased 28.2 % compared to control (without addition).
References:
[1] Chen et al., Bioprocess Biosys. Eng., 40:703–714 (2017).
[2] Burgstaller et al., J. Chem. Tech. Biotechnol., 93:1881-1890 (2018).
For medical biodegradable polyester materials to be used for soft tissue, they must be made in nonwoven or mesh form. There are many ways to make it into nonwoven form, but in order to be used for medical use, it should be a process that does not use chemical additives in manufacturing process and can produce small quantity.
In this study, various processes for the preparation of nonwoven fabrics for soft tissues were studied using biodegradable poly glycolic acid (PGA) and poly lactic-co-glycolic acid (PLGA). The web was well formed in the needle punching process. However, since the strength of the nonwoven fabric is weak and the loss of the process is large, it is considered unsuitable for medical use. The melt blown process was difficult when setting the spinning temperature during manufacture, but it was possible to produce thin, low weight nonwoven fabrics. The flat knitting process is capable of producing meshes with a small amount of 100 ~ 200g of yarn and is suitable for applying expensive biodegradable materials with little loss. In the case of the mesh fabricated by the flat knitting process, it is expected that it can be applied to the part where stretchability is needed in the future because of its excellent stretchability. Circular knitting can produce meshes with a small amount of yarn, and can be manufactured in a thin and light form, but has a disadvantage of curling the cutting portion at the time of cutting. In order to improve the morphological stability, the heat treatment process was introduced and the curling phenomenon was improved and the physical properties were also improved. We have provided a base for manufacturing nonwoven fabrics and meshes for soft tissues that can be used variously according to therapeutic purposes by using medical biodegradable polyester material.
Cataract is caused by the aggregation of crystallin proteins in lens cells of the eye, leading to light scattering and vision impairment. Cataract is known as one of the leading causes of blindness worldwide. Deposition of human γD-crystallin (HGDC), which is a major protein in eye lens, has been linked to cataract. In this study, we used the nuclear magnetic resonance (NMR) spectroscopy in combination with equilibrium chemical unfolding to investigate the structural stability of HGDC. The relatively unstable regions of HGDC were identified by comparing the residual dipolar couplings (RDCs) of HGDC in the environments without and with 5 M urea. Our results suggested that the structure of HGDC is relatively unstable in the regions including residues L92, Q113-R115, and D150-Y151. The structure of HGDC obtained by GeNMR online server was further refined using both residual dipolar couplings results and torsion angles derived from the chemical shift data. Comparison of the structures obtained from different conditions indicated that, relative to its N-domain counterpart, the C-domain of HGDC was more significantly affected by urea. Biophysical characterization of the mutants, which will be obtained by point mutation of the above suggested unstable regions, will be needed for verification.

Chinese Hamster Ovary (CHO) cells are the most commonly used host cell for the production of recombinant therapeutic proteins. In the endoplasmic reticulum (ER) of mammalian cells, ER chaperones are responsible for the folding and assembly of secretory proteins. Soluble ER chaperones have a KDEL (Lys-Asp-Glu-Leu) motif at their C-terminus that is recognized by KDEL receptors. Mammalian KDEL receptors are a family of three members that function to ensure the retrieval of ER chaperones from post ER-compartments back to the ER. In this study, we induced ER stress in CHO cells to investigate its effect on the gene expression level of KDEL receptors and soluble ER chaperones, and whether or not this response is correlated. We also hypothesize that overexpression of KDELR1 could increase the rate of retention of chaperones to the ER; therefore, increase the abundance of chaperones in ER and enhance protein folding and assembly.
The target molecules are the three KDEL receptors [KDELR1, KDELR2 and KDELR3] and KDEL chaperones [Binding immunoglobulin protein (BiP), Calreticulin, endoplasmin (GRP94), and Protein disulfide isomerase (PDI A1)]. KDEL receptors' and KDEL chaperones' gene expression during ER stress induction by tunicamycin were analyzed using Real-Time-PCR (RT-qPCR), KDEL receptors showed less than 2 fold-increase in expression level during ER stress. On the other hand, KDEL chaperones showed several fold increase. This uncorrelated upregulation proposes a possibility of the saturation of ER retention machinery (KDEL receptors), or at least hindered retention of ER chaperones under stress conditions. Recombinant stable overexpression of PDI showed increased extracellular secretion of PDI to the extracellular culture medium, indicating the saturation of ER retention machinery in CHO cells. The stable over-expression of Kdelr1 in recombinant IgG1 producing CHO cells improved IgG1 specific productivity.
Cardiac tissue engineering is an emerging field that holds great promise towards the development of innovative treatment strategies for heart disease. Nanofibrus scaffold act as a 2D surface, due to the lack of cell infiltration and hydrogels, which possess a 3D environment, suffer from lack of good mechanical properties. Therefore, by combining these two types of scaffolds, it would be possible to attain a desired 3D environment with good mechanical properties.
In this study, nanofiber-reinforced composite hydrogels were fabricated by incorporating poly (ε-caprolactone) (PCL)/gelatin nanofibers into chitosan (CS)/heart extracellular matrix (ECM) hydrogels.
To fabricate PCL/gelatin nanofibers, the dual electrospinning technique was used. To fabricate CS/ECM hydrogel, the decellularized heart tissue using appropriate combination of detergents was mixed with CS. After preparing solubilized ECM and CS solution, they were mixed in certain quantities together with specified amount of glutaraldehyde as crosslinker. Then the prepared (PCL)/gelatin nanofibers with specified size were put on the homogeneous hydrogel solution and let the hydrogel solution to penetrate into the nanofibers.
Fourier transform infrared spectroscopy (FTIR) spectra show evidence of intermolecular interactions between the CS and ECM in hydrogel.
To evaluate the physical characteristics of the fabricated nanofibers, scanning electron microscopy (SEM) and contact angle analysis were performed. The mechanical properties of the nanofiber-reinforced composite hydrogels were evaluated using Uniaxial tensile teste and the results indicated that the moduli of the nanofiber-reinforced hydrogels were remarkably higher than those of hydrogel alone. The tensile test results indicate that by varying the ratio of both hydrogel and nanofibers components, moduli between 1 to 3 Kpa and 5 to 100 Mpa could be achieved, respectively. By similar test, the native heart modulus was measured to be about 31 Kpa. By incorporating electrospun PCL/gelatin:CS/ECM (the ratio of 2:1), satisfactory mechanical properties close to that of native heart tissue were obtained.
Achieving precise manipulation of stem cell self-renewal and differentiation behaviors will maximize the potential benefits of stem cell therapy. Here, the multifunctional concept of using robust and chemically defined modifications to support stem cell culture is demonstrated to provide synergistic coupling with concurrently immobilized FGF-2 and chitosan, and the resulting cell culture matrix/interface enables the proliferation of spheroids of human adipose-derived stem cells (ADSCs) with augmented stemness in the form of self-renewal and enhanced (trans-)differentiation potential for diversified cell lineages in the mesoderm, endoderm and ectoderm. The modification and the co-immobilization are performed using a straightforward one-step vapor-based coating technology applicable to a wide range of cell culture materials. The concurrent immobilization of FGF-2 and chitosan revealed an important prospective for designing a material interface that confirms that effective and sustainable factors to determine stem cell fate are obtainable using a facile multicomponent modification approach on culture substrates.