
The bottleneck of realizing a hydrogen energy society is energy loss during hydrogen transport and storage. Ammonia is a promising raw material for hydrogen production because it may solve several problems related to hydrogen transport and storage. Ammonia has four advantages as an energy carrier: (1) Liquefaction is easy. (2) The transport and storage mechanisms have been established. (3) Carbon dioxide is not removed when ammonia is converted to hydrogen. (4) Ammonia has high weight-based and volume-based energy densities as fossil fuel. Hydrogen can be effectively produced from ammonia via catalytic thermal decomposition; however, the resulting residual ammonia negatively influences the fuel cells. Therefore, a high-purity hydrogen production system comprising a catalytic decomposition reactor and a plasma membrane reactor (PMR) has been developed. In this hydrogen production system, ammonia is quickly decomposed into hydrogen and nitrogen in a catalytic reactor, and hydrogen is separated from ammonia decomposition gas by PMR. However, the hydrogen yield of this hydrogen production system was low (16 %). The cause of the low yield was the low hydrogen separation capacity of the PMR with ununiformed plasma firing.
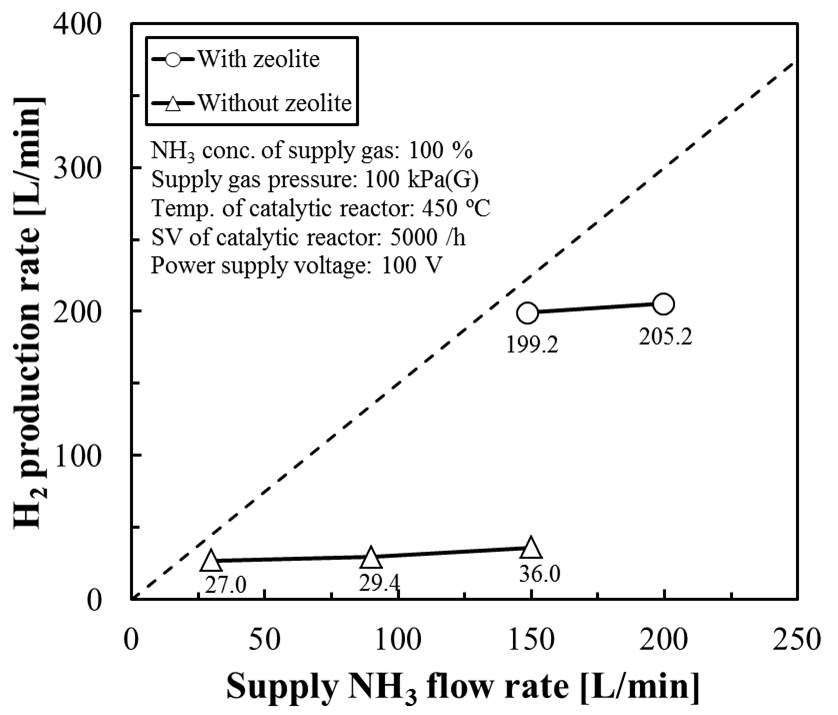
In this study, the state of plasma firing was improved by filling dielectric in the PMR. A columnar zeolite (Tosoh Corporation) was used as a dielectric. In the PMR filled with zeolite, plasma was uniformly fired. By packing the zeolite in the PMR, the hydrogen generation performance of the PMR was also improved. It was also found that the improvement of the production performance is affected by the properties of the zeolite to be filled. The hydrogen refining flow rate obtained 199 L-H2/h from 150L-NH3/h. The hydrogen yield of this hydrogen production system improved from 16 % to 88 %.
We investigated the reaction pathways for reversible formate (HCOO–) dehydrogenation and bicarbonate (HCO3–) hydrogenation on N-doped graphene-supported Pd nanoparticles such as Pd12NC-G, Pd12NC-N1G, Pd12NC-N2G, and Pd12NC-N3G using density functional theory (DFT) calculations, and proposed key factors for the enhancement of the reversible reaction efficiency. In order to achieve this, an anion environment caused by HCOO– and HCO3– was simulated by designing two-sided Pd12NC-G systems with extra electrons. The difficulty of conventional DFT calculations related to simulating the anion environment were overcome by using the two-sided systems. Using the systems, we analyzed reaction pathways for the HCOO– dehydrogenation and HCO3–hydrogenation, and demonstrated that the desorption strength of hydrogen was the potential limiting step for the HCOO– dehydrogenation with the energy barriers as 1.24 (1.49), 1.12 (1.27), 0.76 (0.96), and 1.35 (1.90) eV on Pd12NC-G, -N1G, -N2G, and -N3G, respectively (values for the HCO3– hydrogenation were indicated in parentheses). These results indicate that the adsorption energy of hydrogen can be utilized as a descriptor for the reversible reactions on other surface systems. In addition, we confirmed that the Pd12NC-N2G with the proper amount of N dopants showed optimal hydrogen adsorption strength depending on the smallest d-band center and spin density values, resulting in the lowest energy barriers for HCOO– dehydrogenation (0.76 eV) and HCO3– hydrogenation (0.96 eV). This showed that the appropriate number of nitrogen dopants can provide the optimal balance for the reversible reactions.
Ammonia is a hydrogen storage material that may solve several problems related to hydrogen transportation and storage in the hydrogen society. Catalytic thermal decomposition is a promising technique for producing hydrogen from ammonia. However, Catalytic thermal decomposition of ammonia needs long start-up time for heating reaction field. So, it is not suitable for power generation on site. Cylindrical plasma membrane reactors have been developed to produce pure hydrogen from ammonia. However, the gas flow is not uniform; therefore, the plasma state is unstable, resulting in a low ammonia decomposition rate in the cylindrical plasma reactor. A plasma membrane reactor with a flow channel was considered in order to address this issue, assuming that this configuration would simulate a fuel cell separator to create a uniform gas flow. A 1-mm-wide and 1-mm-deep flow channel was fabricated on the metal plate. 0.1% ammonia gas was supplied to the flow channel plasma reactor at a 0.1-L/min flow rate in an object to test the configuration. Stable plasma was observed; the ammonia decomposition rate reached 20.3 %, which represents a higher conversion efficiency than that measured in a cylindrical plasma membrane reactor with the same gas residence time. Because the plate plasma reactor can be laminated, it can be easily scaled up for large-scale hydrogen production. The effects of the applied voltage, gas flow rates, and ammonia concentration on the ammonia decomposition rate were investigated. The results showed that an increase in the applied voltage leads to a higher ammonia decomposition rate because the high plasma density causes greater dissociation of molecular ammonia by electron impacts. Further, increases in the ammonia gas flow rate or the ammonia concentration reduces the ammonia decomposition rate.
An efficient method for using pulsed plasma to produce hydrogen from ammonia was developed.
To keep the plasma steady under pressurized conditions, internals such as zeolite particles was available. Actually, hydrogen production rate from ammonia was increased by the packed bed plasma membrane reactor. This report discusses the cause of the increase of hydrogen. The V-Q lissajous and fundamental characterization of the particles was investigated for some kinds of zeolite particles. It seems that the dominant factor is H radical generation by the zeolite.
The technical issue of hydrogen, which is used for fuel cell electronic vehicle, is the storage and the transportation. Some chemicals, i.e., 2-propanol and methyl-cyclohexane, have potential as a carrier of hydrogen due to their advantages such as liquid phase at room temperature, lower toxicity, etc. The conventional hydrogen production process from these “hydrogen carrier” was complex and energy-wasting because the process consists of (1) dehydrogenation reactor (endothermic step), (2) condenser of an organic compound (exothermic step), and (3) mechanical compressor.
We propose a new electrochemical device which able to produce a proton, separate hydrogen, and compress hydrogen in one step. The setup of the device is similar to the polymer electrolyte fuel cell (PEFC). The proton forms at anode, hydrogen gas at the cathode is separated from the organic compounds by the polymer electrolyte membrane, and hydrogen is compressed by the conversion from electronic potential to chemical potential (Nernst equation).
In this work, we developed the electrochemical device to obtain pressurized hydrogen from 2-propanol. The one-pass conversion and obtained pressure were measured during constant voltage operation.
We prepared a PEFC-like device with Pt/C catalyst (for anode and cathode) and Nafion 117 membrane. A hydrogen carrier, 2 M 2-propanol, was supplied to the anode. The –1 V of voltage was applied to the device, the one-pass conversion was evaluated from the composition of anode effluent, and cathode gas pressure was measured by a pressure transducer.
The obtained one-pass conversion was ca. 8% at –1 V, and cathode gas pressure increased 0.9 bar during 1 h. The major component in cathode gas was hydrogen, in the meanwhile, some impurities, i.e., 2-propanol, acetone, and methane, were found. Methane is considered to be formed from acetone and hydrogen with Pt catalyst.
Plasma membrane reactor has been developed to produce hydrogen from ammonia. A cylindrical type reactor is available for hydrogen production; however, a plate-type reactor is desired for a mobility use. A plate type plasma membrane reactor has made by a flow channel plate and original electrode, which shows stable plasma firing by dielectric barrier discharge. Effect of membrane thickness on hydrogen permeation characteristics was examined, and its characteristics were compared with the cylindrical plasma membrane reactor.