The equilibrium diagrams for the decomposition of magnesium alanate (Mg(AlH4)2) nanoparticles were constructed as a function of particle size and temperature to better understand nanostructuring of the complex hydrides for hydrogen storage. Relatively smaller nanoparticles of Mg(AlH4)2, MgH2, and Al ranging from 1 to 2 nm were directly calculated by using density functional theory (DFT) calculations, and cluster expansion and Monte Carlo simulation methods were developed to predict the free energy contours of 1 ~ 50 nm nanoparticles. Our prediction demonstrates that bulk Mg(AlH4)2 can release hydrogen but its uptake reaction is unfavorable, and bulk Mg(AlH4)2 is metastable with respect to bulk MgH2. However, in the cases of nanoparticle systems, hydrogen release and its recharging may be possible by controlling the particle size and temperature, which may facilitate experimental studies to determine the thermodynamically favored reaction pathways for the dehydrogenation and hydrogenation processes of Mg(AlH4)2 nanoparticles. We also provide the equilibrium diagrams for Mg(AlH4)2 nanoparticle decomposition depending on a hydrogen partial pressure.
Solid alkaline fuel cells (SAFCs) have attracted much attention as next-generation energy conversion electrochemical devices. However, practical applications of these devices are limited because of the drastic decrease in the cell performance during the high temperature operation, under alkaline condition. This low performance of the device is mainly attributed to the severe degradation of membrane–electrode-assembly (MEA). Therefore, the development of a durable and stable MEA is critical to improve the cell performances and stabilities in this system.
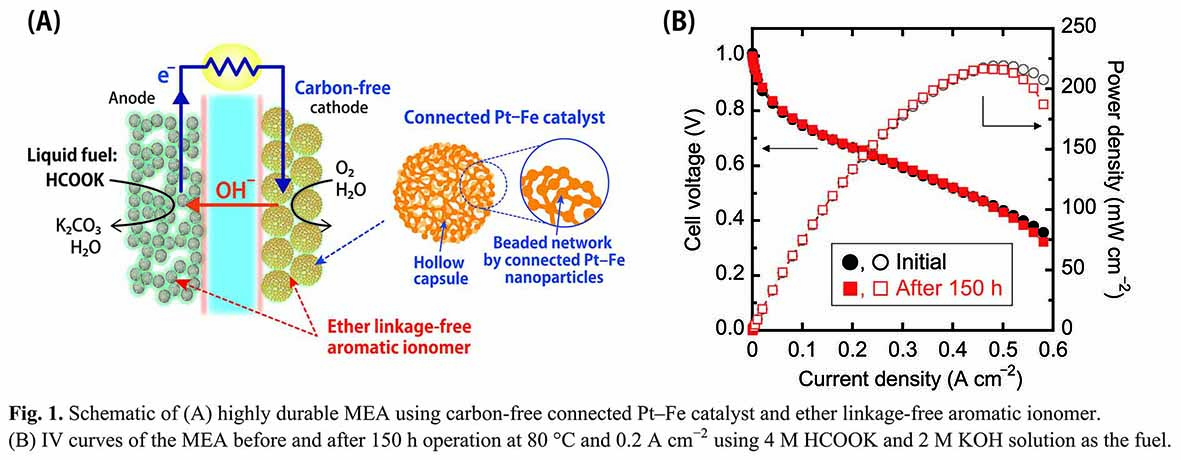
Here we report the development of a highly durable MEA, as shown in Fig. 1A, using a carbon-free cathode catalyst [1,2] and an aromatic polyelectrolyte without ether linkage [3]. The carbon-free catalyst consists of a nanosized beaded network formed by the connection of Pt–Fe nanoparticles. The beaded metal network is electrically conductive, enabling the removal of carbon support from catalyst layers. Hence, carbon corrosion problems can be avoided, leading to high durability against the cyclic start-stop operation of the system.
The fabricated MEA, operating at a high temperature of 80 °C, exhibited high power density of 220 mW cm–2 and high open-circuit voltage of about 1.0 V (Fig. 1B), wherein, a mixture of aqueous solutions of 4 M HCOOK and 2 M KOH was used as the fuel. Further, more importantly, the high performance of the MEA was retained even after the operation at 80 °C and 0.2 A cm–2 for 150 h. Thus, for the first time, we demonstrated a highly durable and stable MEA in alkaline medium for the direct formate SAFCs.
[1] T. Tamaki, H. Kuroki, T. Yamaguchi et al., Energy Environ. Sci., 8, 3545–3549 (2015).
[2] H. Kuroki, T. Tamaki, T. Yamaguchi et al., ACS Appl. Energy Mater., 1(2), 324–330 (2018).
[3] S. Miyanishi and T. Yamaguchi, J. Mater. Chem. A, 7, 2219–2224 (2019).
Methanol synthesis from CO2 hydrogenation has been recently an object of study because CO2 is a potential feedstock based on the concept of a methanol-based economy. However, the commercial catalyst Cu/ZnO/Al2O3 does not show enough activity and selectivity of the CO2 hydrogenation. Hence, it is important to develop suitable catalysts for the CO2 hydrogenation. So far, we reported that Cu/ZrO2 catalysts hydrogenate CO2 selectively to methanol. In this study, we focused on flame spray pyrolysis (FSP) which are widely used for producing nanosized metals/metal oxides on an industrial scale. One of the advantages of FSP is the formation of small metal nanoparticles at extremely high metal loading on metal oxide particles. This time, we tried to prepare highly loaded Cu nanoparticles on ZrO2 (20-80 wt%_Cu) by the FSP technique and investigated the effect of Cu loading on activity and selectivity of CO2-to-methanol hydrogenation. When Cu loading was 60 wt%, the catalysts showed higher methanol yield and methanol selectivity than did a commercial catalyst Cu/ZnO/Al2O3. Its catalytic performance was derived from the unique structure: Cu nanoparticles (10-20 nm) were deposited on ZrO2 nanoparticles (5-10 nm). In addition, the Cu nanoparticles were strongly interacted with the ZrO2 nanoparticles, leading to electron transfer from Cu to ZrO2. The interaction might create specific active sites for the CO2 hydrogenation.
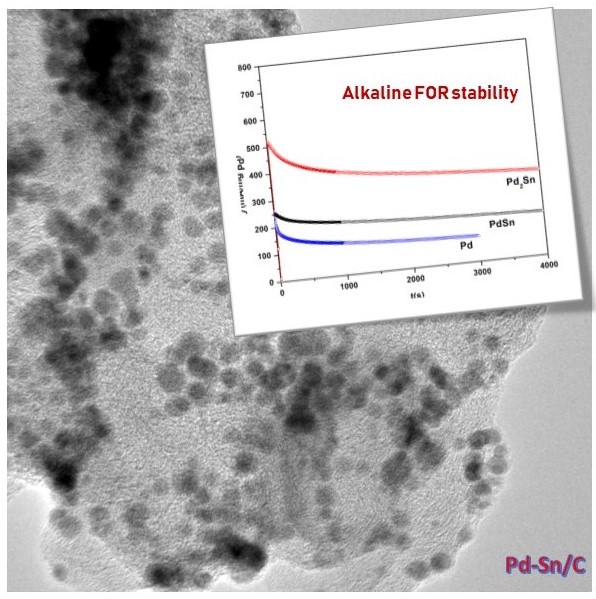
Direct Formate Fuel Cells (DFFCs) hold a key stake in the energy systems looked into for answers to the increasing futuristic energy demand, largely due to the offered potential practical energy generation capability along with the safety, low cost and environment friendliness compared to the counterpart alcohol fuels (DEFCs & DMFCs). Palladium has shown the most promising results in recent years for enhanced liquid-fuel oxidation kinetics that could effectively replace platinum in terms of performance and extensive application span, particularly in alkaline medium. In our study, we engineer alloys based on Pd and transition metal (Pd-Ni) /post-transition metal (Pd-Sn) over carbon support using an exclusive wet chemical technique. The formed alloy compositions showed overall enhanced formate oxidation half-cell performance and longer stability compared to Pd/C in alkaline medium. The performance analysis based on the structural and surface characteristics of these nanoalloys obtained over carbon support is elucidated and presented. The successfully developed catalyst compositions and technique employed paves way for a scalable strategy for realizing highly efficient anode catalysts for DFFCs.
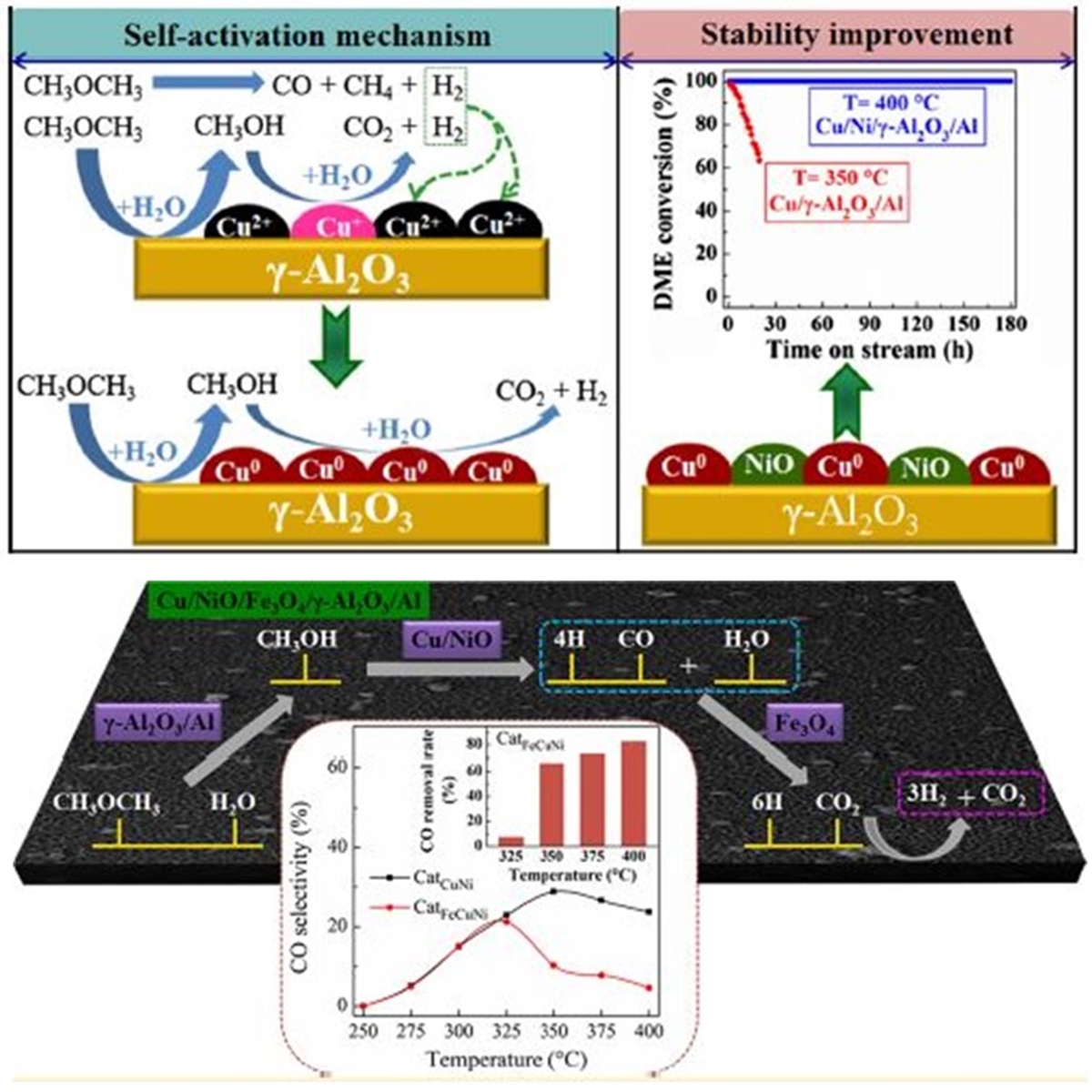
The depletion of fossil fuels and increasing energy demand has driven the research and exploration of new energy alternatives. Hydrogen is considered to be a clean source of energy and steam reforming of dimethyl ether (DME) is regarded as a promising hydrogen production process for fuel cell applications. A plate-type anodic alumina supported Cu composition catalyst was developed to investigate its catalytic performance in steam reforming of DME. It is found that the fresh Cu/γ-Al2O3/Al catalyst without H2 pre-reduction treatment exhibited the similar catalytic activity as the pre-reduced one. The XPS results show that Cu+ species exist in the surface of the fresh catalyst, which results in the self-activation reaction. However, the Cu/γ-Al2O3/Al catalyst showed a quick deactivation at 350 °C due to the sintering of copper. As an approach, a second component Ni was doped to the Cu-based catalyst. Furthermore, the effect of nickel loading and chemical state on the activity of catalyst was extensively investigated. The proper amount of nickel doping is helpful in improving the dispersion of copper species, and thus enhancing the catalytic activity. Finally, a 180 h stability evaluation was carried out and the results show that the optimized Cu/Ni/γ-Al2O3/Al catalyst has an excellent stability under critical conditions with 400 °C, and gives a 100% DME conversion. However, a high CO concentration (ca. 26%) was detected. As such, a multifunctional catalyst combined DME SR and high temperature water gas shift reaction (HT-WGSR) was developed. The CO reduction mechanism was proposed over the Fe-doped Cu-based multifunctional catalyst. Furthermore, the effects of iron loading on the physicochemical properties and performance of catalysts were extensively investigated. The results show that the proper amount of iron doping was helpful in improving the dispersion of Cu, and thus enhancing the catalytic performance and decreasing CO concentration. Finally, it is found that the optimized Cu/Ni/Fe/γ-Al2O3/Al multifunctional catalyst has excellent stability during a 200 h test, and gives a 100% DME conversion at 400 °C in both the microreactor and fixed-bed reactor. Furthermore, crossflow channels and parallel-flow channels were built respectively by mesh-type catalyst and plate-type catalyst in the microreactor. The gas/solid mass transfer limitations in both flow channels were investigated by Damköhler number (Da). The results showed that the Da values of cross flow were always < 0.01, while the values of parallel flow were > 0.01. It indicated that the diffusion over mesh-type catalyst was less influenced by temperature and reactor height, making it a more appropriate choice for the microreactor.