
One of the issues for commercialization of direct methanol fuel cell (DMFC) is the slow kinetics of the anode reaction, and an alternative catalyst with high activity has been desired. PtRu nanoparticles supported on CeO2 nanoparticles embedded carbon nanofibers (CECNF), PtRu/CECNF, showed two to three times higher methanol oxidation reaction (MOR) activities due to the support effect of CECNF which efficiently develops the positive interaction between Pt and CeO2 by the CeO2 embedding fiber structure. The interaction can be explained by the reaction mechanism with an oxygen vacancy of the CeO2. In this study, the ion-beam irradiation was employed to introduce oxygen vacancies to the support material in order to increase the MOR activity of the catalyst.
A sheet of CECNF was prepared by using the electrospinning technique. To the CECNF sheet, irradiation of high-energy ion-beam, Ar9+ 127 MeV, was conducted at the different fluence (ion-beam density based on subjected area). After that, PtRu nanoparticles were deposited on the CECNF by a microwave polyol method. The catalytic activity for methanol oxidation reaction was evaluated by using a three -electrode cell with a glassy carbon electrode as a working electrode.
It was found that the MOR mass activity of the PtRu/CECNF increased, up to about 25%, with the increase of the fluence up to a certain value, then decreased with further increase of the fluence as shown in Table 1. The crystalline size of CeO2 in PtRu/CECNF, obtained from XRD profile using Scherrer's equation, decreased with the increase of fluence. EDX results elucidated a decrease of oxygen content in the catalyst due to the irradiation. These results suggested that the ion-beam irradiation destroyed a part of the crystal structure of CeO2, and it enhanced the MOR activity at the proper fluence.
Recent rapid decline in the price of renewable energy increases the importance of energy carriers. Formate solution is one of the envisaged candidate for liquid energy carriers, since formate can be electrochemically regenerated in high energy efficiency from carbon dioxide. For the use of formate solution as an energy carrier, formate needs to be converted to electricity with high energy efficiency.
Our aim in this study is to clarify design strategy of electrocatalysts for formate oxidation reaction (FOR) in alkaline conditions. Palladium (Pd) is known to show better activity for FOR in alkaline solution than platinum. The reaction mechanism of FOR on Pd in alkaline condition remains controversial; although many groups suggested a reaction mechanism without any poisoning species (strongly bound species) such as carbon monoxide (CO), a few studies showed the presence of bound CO. If CO exists, the FOR activity of Pd can be improved by reducing CO adsorption on its surface, which is possible by incorporating oxophilic materials such as ceria (CeO2) and ruthenium (Ru).
Herein, we sequentially deposited CeO2 and Pd over carbon support (Pd/CeO2-C). This Pd/CeO2-C showed better activity for FOR in alkaline medium than Pd/C. The result of an in-situ x-ray absorption spectroscopy (XAS) measurement at Pd K-edge showed the formation of CO on Pd in the presence of formate. Also, the comparison of the spectra between Pd/CeO2-C and Pd/C showed a decrease in CO adsorption with the concomitant increase in OH adsorption on Pd/CeO2-C. We then synthesized Pd-Ru alloy to more effectively incorporate oxophilic materials with Pd. PdRu/C showed better activity than Pd-CeO2/C and Pd/C. These results suggest that the incorporation of oxophilic materials improves the FOR activity on Pd-based catalysts in alkaline medium by reducing the CO adsorption.
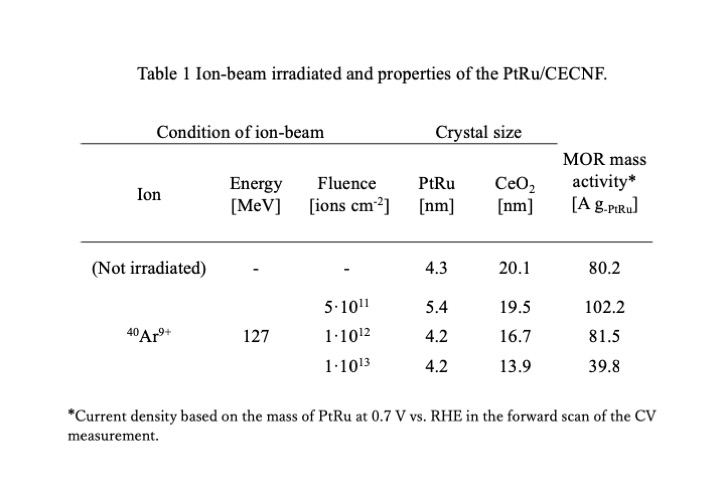
At present, polymer electrolyte fuel cell (PEFC) technology is supposed to be commercialized in a wide range. However, water management is an essential problem to achieve maximum performance. The relative humidity (RH) of gases should be controlled to prevent membrane dehydration and liquid water flooding to maintain a high performance level. Hydrogen recycling is one of methods to humidify the cell with water produced in redox reaction, while the overall hydrogen utilization can be 100%. In this study, water transport in a PEFC system with hydrogen recycling was analyzed by combining plug flow reactor model and dimensionless modulus model in the through-plane direction in steady state, when the dry hydrogen and humidified air are fed to an 80 °C isothermal cell. The gas channel was regarded as a straight line in the model. Although the water flux through proton exchange membrane (PEM) has different distribution at different current density, total water permeation rate is 0, as far as the outlet gas of anode recycles to the inlet entirely (Fig. 1). In the case of co-current which the gas flows have the same direction on the anode and cathode sides, current density as well as electroosmosis increases from inlet to outlet of gas channel in low current region, because of humidification by produced water, which enhances the proton conduction in PEM and cathode layer. Besides, the difference in RH of gas flow on two sides of PEM creates water diffusion. When the gas flowrate increases, decreasing proportion of produced water makes PEM dehydrated, and the performance deteriorated (Fig. 2). The RH in the cell increases with decrease in flowrate. At 1 A/cm2 and H2/O2=2, RH becomes 68% at the exit of the cathode in case of 300 mL/min oxygen and RH reaches 100% at 21 mL/min.
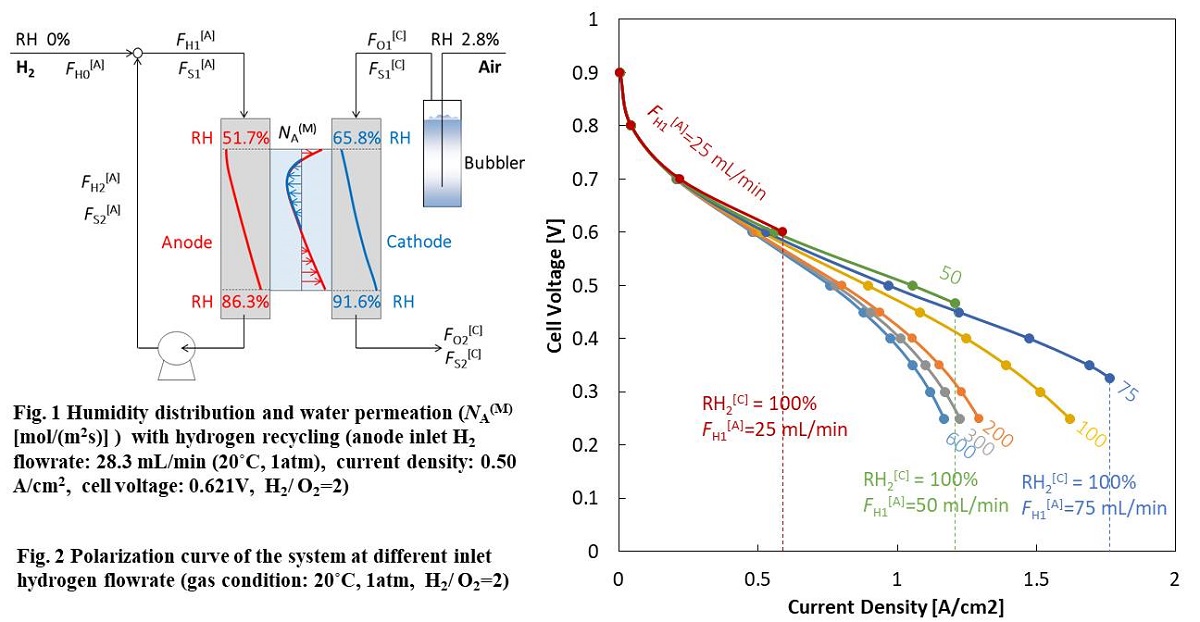
The use of bimetallic nanoparticles (NPs) reduces the noble metal usage without sacrificing the electrocatalytic activity. To theoretically investigate the influence of the NPs' properties, such as strain, stability, or electronic structures on the catalytic activity requires the use of a “close to real size” cluster models. In this study, the phase stability, its origin and electronic structure of Pt3M (M = Co, Ni, and Cu) are investigated using density functional theory (DFT) method. Bimetallic Pt3M-NPs containing 201 (Pt150M51), 405 (Pt303M102), and 711 (Pt533M178) atoms in total were modeled considering three different configurations; 1-skin layer (S1), 2-skin layers (S2), and solid solution (SS). The stability of the Pt3M-NPs was approximated by the excess energy, which contains the mixing enthalpy and configurational entropy. In all cases, the bimetallic NPs were more stable than the monometallic Pt-NPs. For the S1 systems, the mixing enthalpy is the dominant term, and the configurational entropy value is the smallest compared to SS, and S2 systems, as shown in Figure 1. Electronic equilibration in line with the work function difference between Pt and M led to charge transfer from the M to Pt, reducing the surface Pt atoms in the S1 configuration. Additionally, the presence of M of smaller atomic radius than Pt in the core of the nanoparticle made the surface atoms more relaxed compared to the monometallic system, thus decreasing the stress in the structure and helping to accommodate the extra electrons from the M in the subsurface. These changes in the electronic structure and geometrical features were used as descriptors of the Pt3M stability. For Pt3Co and Pt3Ni the stability is largely determined by the surface charge, while for Pt3Cu the dominant factor is the surface strain.
Polymer Electrolyte Fuel Cell (PEFC) is desired to be operated at high temperature such as 90 °C for stationary applications during the period from 2020 to 2025 in Japan. It can be expected thinner polymer electrolyte membrane (PEM) and gas diffusion layer (GDL) would promote the power generation performance of PEFC at this temperature. However, the current PEFC has Nafion membrane and is usually operated within the temperature range between 60 °C and 80 °C. The aim of this study is to clarify the impact of thicknesses of PEM and GDL on temperature distribution in single PEFC generated at high temperature such as 90 °C and to propose the optimum components combination.
As a result, from the investigation of impact of thickness of GDL on the power generation characteristics using the thickest PEM, Nafion 115, the impact of relative humidity of supply gas on the power generation characteristics was small for thin GDL. In addition, in-plane temperature distribution from the inlet to the outlet was flat by promotion of water transfer when using thin GDL. On the other hand, from the investigation of impact of thickness of PEM on the power generation characteristics using thin GDL, i.e., TGP-H-030, the impact of relative humidity of supply gas on the power generation characteristics was not acknowledged. However, the power generation performances using Nafion 212 and Nafion 211 were higher than that using Nafion 115. As to in-plane temperature distribution, it was flat when using Nafion 115 and Nafion 211, while it was increased from the inlet to the outlet using Nafion 212.
It was revealed that the thinnest combination of Nafion 211 and TGP-H-030 was the optimum for high temperature operation such as 90 °C from the viewpoint of the power generation performance as well as controlling in-plane temperature distribution.