
Polymer electrolyte fuel cell (PEFC) has been attracting attention as a highly efficient power generation system. Since Pt used in a catalyst is scarce and expensive, it is important to develop a low-Pt catalyst. A core-shell catalyst, which consists of thin Pt shell on non-Pt metal core, has been developed to reduce the amount of Pt. Typically, Cu-UPD (underpotential deposition) followed by surface limited redox replacement (SLRR) is used to fabricate the core-shell catalysts. We have been developing a novel method suitable for mass production of membrane-electrode assembly (MEA) by initially fabricating a catalyst layer (CL) from the core material, and then perform Cu-UPD and SLRR on the CL to obtain a new CL of core-shell catalyst. Moreover, we focused on nanosheet (ns) catalysts. A general Pt-supported carbon catalyst has a problem in durability, since small Pt particles are easily aggregated and dissolved due to its large surface energy. It was reported that Ru@Pt-ns/C catalyst using Ru-ns as the core and Pt as the shell, exhibits high specific activity and high durability due to the low surface energy. In this study, a novel method to produce an electrode with low-Pt core-shell nanosheet catalyst was developed by using Cu-UPD/SLRR in the CL.
The effect of the ionomer ratio on the formation of core shell was investigated. Metallic luster was observed on the surface of the CL for I/C = 0.5 and I/C = 1, indicating abnormal deposition of Pt. However, it was not observed for I/C = 0.25, suggesting uniform core-shell formation. The influence of the number of times of Cu-UPD/SLRR was investigated. The Pt mass activity was low when 2 cycles of Cu-UPD/SLRR were applied but it improved when 4 cycles were applied. The Pt mass activity of Ru@Pt-ns/C prepared by the novel method was higher than Pt/C.
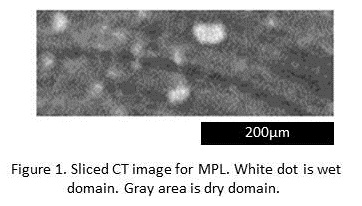
It is well known that laminating a microporous layer (MPL) to substrate such as carbon paper prevents from flooding in catalyst layer (CL) for polymer electrolyte fuel cell (PEFC). It is therefore important to visualize 3D water distribution in both the MPL and substrate to design the material. Visualization of 3D water distribution in the substrate was conventionally achieved by operando X-ray Computed Tomography (CT). However, 3D water distribution in the MPL has not been visualized due to the strong X-ray adsorption of the Pt in the CL. Here we present new method for visualizing 3D water distribution for the MPL. In the experiment, water vaper was diffused in the MPL, and its condensation process was measured by X-ray CT. This method does not need the CL. Conventional problem strong X-ray adsorption of the CL for Operando-CT can be avoided. Obtained sliced image of MPL is shown in Figure 1. Water in the MPL is observed as polka-dot patterns. This suggests that pore to water drain and pore to gas diffusion is separately exists in the MPL. In addition, water movement from wet domain from the MPL to substrate was visualized. These results show potential of the method to understand water condense and drain phenomena in the MPLs.
Optimization of the PEFC design is a crucial subject for its deployment and production expenses. However, a comprehensive understanding of its behaviour is needed, including its own structure, electrochemical reaction, reactants and products mass transfer limitations concerning proton and oxygen in principal. The mass transfer takes place through electrostatic drag for proton, and convection and diffusion of oxygen in the cathode catalyst layer (CCL). The authors introduced four dimensionless moduli which govern behavior of CCL.
Effectiveness factor is one under the reaction rate controlled regime, yet, is inversely proportional to Thiele modulus, as far as the convective flow effects are negligible and under oxygen transport control conditions. Under proton transport control conditions, the effectiveness factor is inversely proportional to the modulus that represents the ratio of the reaction rate constant to the effective proton conductivity.
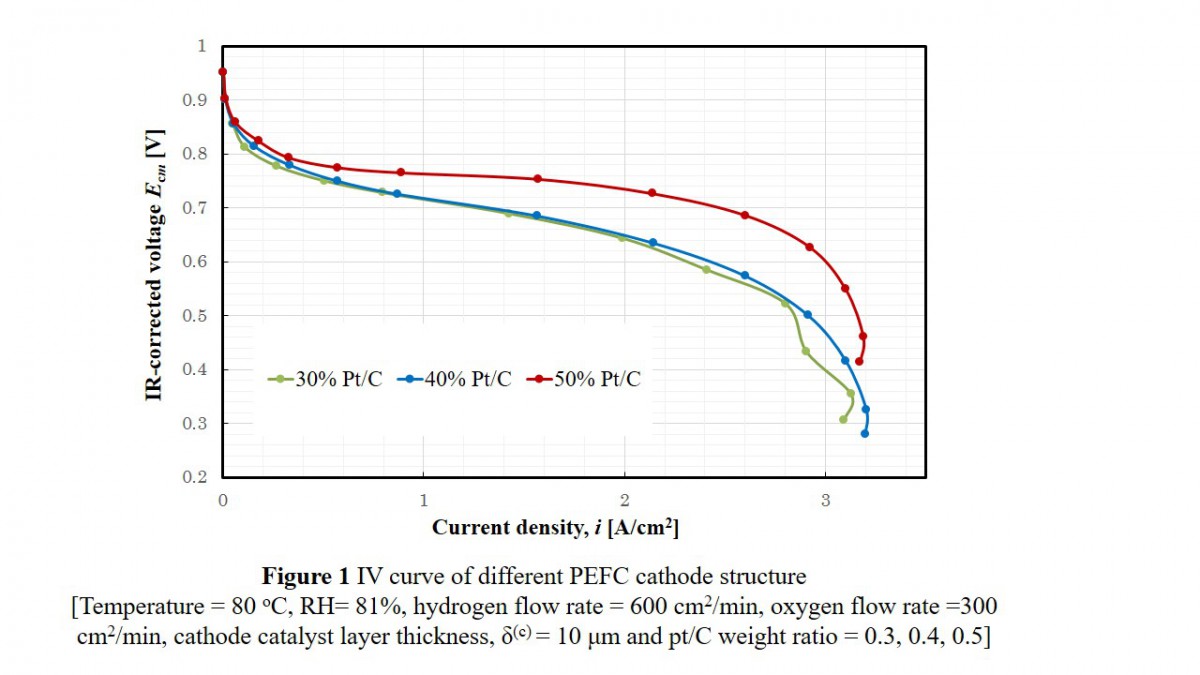
To determine the effectiveness factor and dimensionless moduli, experiments were done on NR-212 (50 μm) with Pt/C weight ratio of 0.3, 0.4, and 0.5. For each Pt/C weight ratio, cathode catalyst layer thickness was 10, 30, 50 μm, using electrochemical measurement system. The impedance spectra were measured in a range of frequency from 10 mHz to 100 kHz.
Figure 1 shows the relation between IR-corrected cell voltage and current density for 10 μm cathode catalyst layer having a Pt/C ratio of 0.3, 0.4 and 0.5. The overall mass transfer limitation between Pt/C = 0.3 and 0.4 was comparable. Oxygen and proton transfer resistances were estimated.
Solid Oxide Fuel Cells and Solid Oxide Electrolysis Cells (SOFCs / SOECs) have much attention because SOFCs have the advantage of being able to convert fossil fuels to electricity with the highest efficiency and SOECs have the advantage of requiring less power for electrolysis compared to PEMs due to high temperature operation. From these backgrounds, both have been considered for practical use.
In recent years, studies on reversible Solid Oxide Cells (R-SOCs) which means a combination of SOFCs and SOECs are in progress. R-SOCs can be regarded as an energy storage system similar to a battery, but have the drawback of a smaller conversion efficiency than that of a battery. In this work, we performed theoretical efficiency calculations of R-SOCs system using an oxide-ion conductor and a proton conductor to show the merit in comparison with a battery.
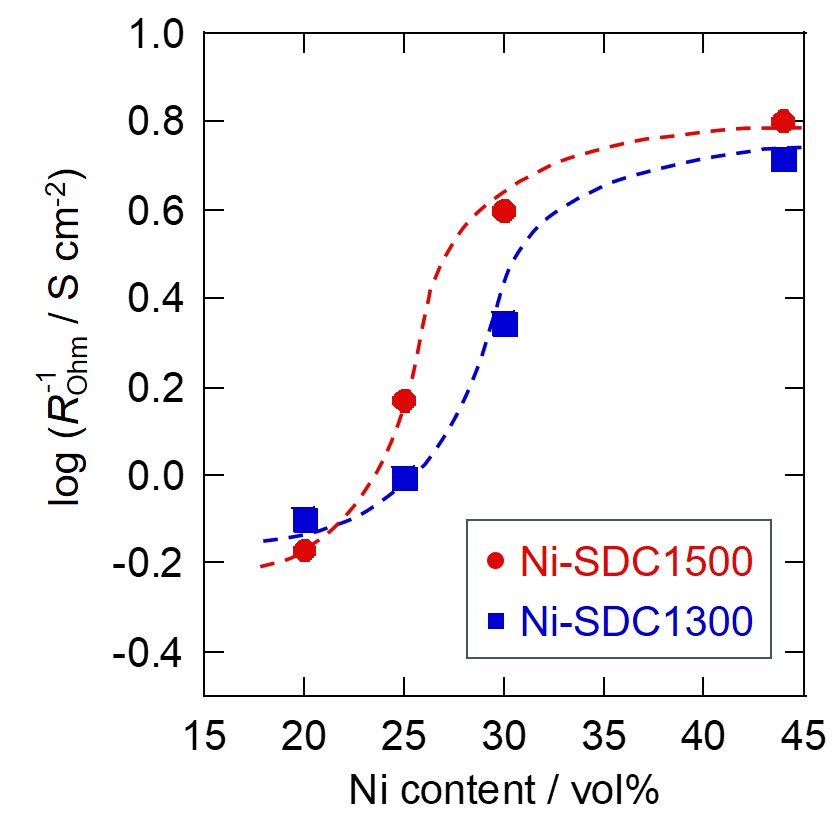
Ni-based cermets are typically used for anodes of solid oxide fuel cells. However, the Ni phase in such anodes can cause durability problems. One possible approach for mitigating the problems is to reduce the Ni content in the anode, although decreased Ni amount in turn reduces conductivity and electrochemical activity of the anode. In this study, Ni-SDC (samarium-doped ceria) cermet anodes were prepared with different SDC particle sizes and Ni contents, and it was investigated how particle size ratio of the SDC and Ni affected the performance at low Ni contents. Ni-SDC|YSZ|LSM cells were fabricated, then current-voltage characteristics and AC impedance spectra were measured. Impedance analyses with equivalent circuits revealed that ohmic resistance was the primary factor affecting the anode performance in the tested system, and it was found that large SDC particles were effective in reducing the ohmic resistance even at low Ni contents. To further investigate the effects of the particle size ratio, conductances of the Ni-SDC anodes with different SDC particle sizes were calculated from the measured ohmic resistances. The conductances decreased sharply at certain volume fractions of Ni, and the threshold volume fractions shifted toward lower Ni contents when large SDC particles were used. Independently, threshold Ni volume fractions were calculated according to percolation theory, using particle sizes of Ni and SDC estimated from SEM observations. The thresholds thus calculated were in good agreement with those obtained from the ohmic resistance, indicating that the electrical conductivity of the Ni-SDC anodes was governed by the formation of segregated and conductive Ni clusters.
Due to the expanding installation of renewable energy, to solve the gap between the unsteady power supply and the demand becomes a key issue, and the reversible solid oxide fuel cell/ electrolyzer (rSOFC/EC) are increased their attention as a large-scale energy storage. To control their unsteady operations, the quantitative understanding of the reversible reaction kinetics is required. A number of reaction kinetics models of SOFC on Ni/ yttria stabilized zirconia (YSZ) have been proposed [1-3]. The rate determining was described as a surface chemistry on triple phase boundary (TPB) under the local equilibrium between oxygen, O2- and electron [1, 2], or the charge transfer reaction of neutral absorbent, surface ion and electron between Ni and YSZ [3]. In both case, the reaction of SOFC/EC have not been successfully described as a unified kinetics.
We applied the existing reaction model of SOFC anode, which we previously developed [2], to the reversible SOFC/EC with the series of H2/H2O gas components to discuss the SOEC anode reaction. The model is described the kinetics by competitive adsorption reaction at TPB with oxygen activity (aO) calculated from anode potential. As a result, the current density of SOEC was much larger than the extrapolated value by SOFC data at any H2/H2O gas condition. By reinvestigating the local equilibrium of adsorbents, oxide ion and electron on Ni, we proposed a modified kinetics model by defining the electron activity ratio as another independent variable. The kinetics is discussed with the shift of local oxygen equilibrium by changing the charge balance.
Acknowledgement: a part of this work is supported by the New Energy and Industrial Technology Development Organization (NEDO)
[1] Mizusaki et al., Solid State Ionics, 70/71. 52 (1994).
[2] Ihara et al., J. Electrochem. Soc., 148(3), A209 (2001).
[3] Bieberle et al., J. Electrochem. Soc., 148(6), A646 (2001).