Due to population and economic growth, further increases in energy demand are predicted globally. Therefore, the construction of a sustainable carbon cycle system is essential to address fossil fuel depletion. The conversion of carbon dioxide (CO2) to hydrocarbons is currently a major challenge.
Among available technologies, the electrochemical conversion of CO2 on copper (Cu) electrodes has gained popularity as a novel technique to realize a sustainable carbon cycle because of improvements in power generation using renewable energy sources, such as solar and wind energy. However, the electrochemical conversion of CO2 on Cu electrodes not only produces valuable hydrocarbon products but also emits byproducts such as carbon monoxide (CO) and hydrogen (H2) because the product selectivity of this conversion is sensitive to the Cu electrode's surface properties, such as atomic arrangement and morphology. Therefore, electrodes for a more selective electrochemical conversion of CO2, which promote hydrocarbon formation and suppress byproduct formation, are required for practical technology.
In this work, we report the selective electrochemical conversion of CO2 on nickel (Ni) / Cu binary electrodes. The formation of hydrocarbons on these electrodes was strongly dependent on both the crystal structure of the supporting Cu electrode and the amount of Ni deposition. This suggested that the adsorption of a carbonate intermediate on the surface of Ni/Cu binary electrodes, formed by the electrochemical conversion of CO2, improves to the selective electrochemical CO2 conversion for hydrocarbon formation compared with pristine Cu electrodes. Based on these results, we discuss the mechanism of the selective electrochemical CO2 conversion and the inhibition of H2 byproduct formation on Ni/Cu binary electrodes.
Formic acid or formate is considered as a perfect fuel for clean energy generation through fuel cells. Formate can be electrochemically generated from CO2/HCO3–. According to techno economic analysis, formic acid/formte is one of the most profitable products of electrochemical CO2 reduction reaction. Sn based electrocatalysts are known for the selective production of formate from CO2 with a faradaic efficiency >80%. But main drawback of these catalysts is high overpotential for the reaction. Various methods have been tried to reduce the overpotential and to improve the catalytic activity of Sn. In this work, we investigated the effect of Pd doping on the surface of Sn for the electrochemical generation formate from CO2 and HCO3–.
We prepared Sn and Pd nanoparticles decorated N doped carbon fibers (SnPd-NCF) from SnCl2, PdCl2 and polyacrylonitrile using electrospinning method. SEM and TEM analysis confirm the uniform distribution of Sn and Pd nanoparticles over N doped carbon fibers. XPS spectra confirmed nitrogen-doping on carbon fibers. We found that an addition nearly 1.5 wt % of Pd on Sn surface greatly enhance the faradaic efficiency of formate formation and reduce the overpotential nearly by 0.3 V compared to that of Sn nanoparticles decorated N doped carbon fibers (Sn-NCF).
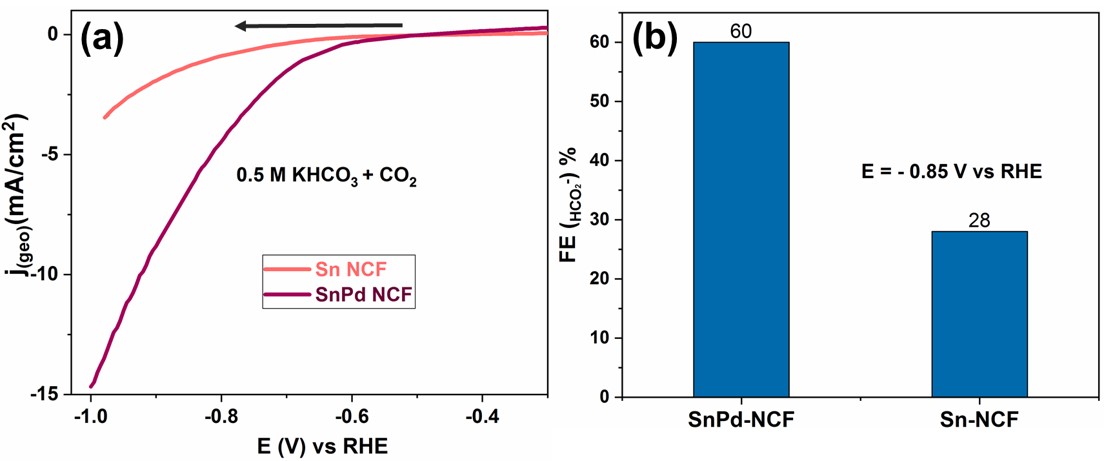
Figure. (a) Voltammetric response of CO2 reduction on Sn-NCF and SnPd-NCF in 0.5 M KHCO3 solution saturated with CO2. (b) Faradic efficiency of formate produced on Sn-NCF and SnPd-NCF during CO2 reduction reaction at -0.85 V vs RHE.
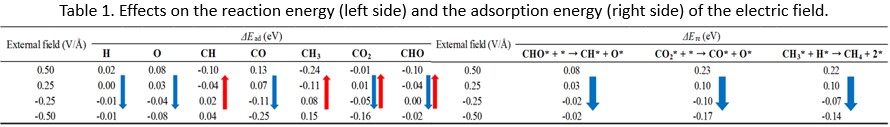
Non-Faradaic electrochemical modification of catalytic activity (NEMCA) by impression of electric field (EF) is one of the methods to improve catalyst performance. It has been discussed that oxygen anions forced electrochemically to adsorb on catalyst surface alter the catalyst electric property. However, given EF also changes the catalyst electric property directly. Because it is difficult to divide down these effects with an experimental approach, we have tried to theoretically investigate the mechanism of NEMCA in CO2 methanation (CO2 + 4H2 → CH4 + 2H2O (1)) in solid oxide electrolysis cell (SOEC) using the density functional theory (DFT). In concreate, we have focused on the rate-determining steps (RDSs) of CO2 methanation proposed by our detailed reaction mechanism analysis. We have calculated the adsorption energies of hydrocarbon species related to RDSs on Ni(111) (CO2 → CO + O (2), CHO → CH + O (3), CH4 → CH3 + H (4)) with EF or co-adsorbed oxygen atoms. In our calculative setup, used is the model sandwiched between thin film condenser boards connected to the outlet electrode for direct EF impression calculations, While used is the Ni(111) surface with different number of oxygen atoms for co-adsorption calculations. Due to space limitation, just an example of our calculations is shown in this abstract. With external EF, the equilibriums in reactions (2), (3) and (4) are all leaned to products side in the EF from the gas phase to the Ni surface (negative values in Table 1), which means CO2 methanation is promoted in this EF direction. This tendency is caused by the destabilization of reactants (CH3 in reaction (3)) or the stabilization of products (O, CO in reactions (1), (2)) with an EF as shown in Table 1 (right side). Other results and detailed discussion will be reported at our presentation in this conference.
Electric double layer capacitor (EDLC) can store energy by an electric double layer at the interface between the electrode and the electrolyte, which is one of the emerging energy storing devices. High capacity, high durability and rapid charge-discharge capability are attractive, however, low energy density poses drawback to practical use. Specific surface area, surface functional group, and pore size distribution are important controlling factors to improve energy density. In this study, the relationship between the surface functional group and the pore size distribution, and the EDLC capacity was investigated while the specific surface area was nearly equal value as 1200±100 m2/g. In addition, the effect of particle was investigated.
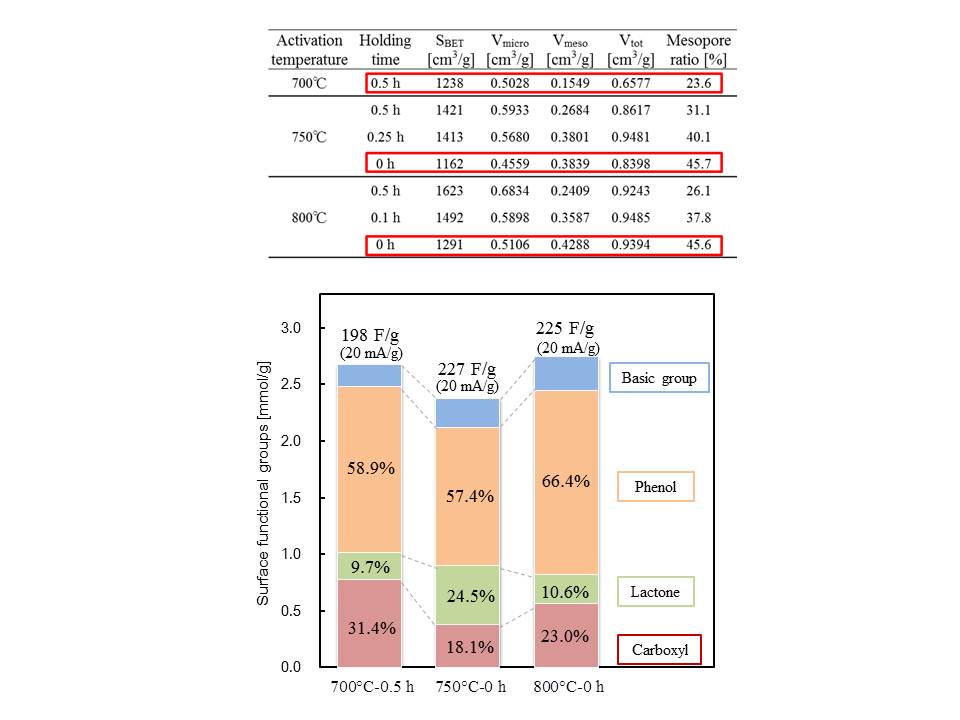
We prepared activated carburized furfural resin particles (1 μm in diameter) with KOH activation for 0 to 0.5 h at 700 to 800 °C in N2 flow, then investigated the electrostatic characteristics of the capacitors in 6M KOH by using a two coin-shaped electrodes. Activated carbon (1 μm in diameter) treated by 750 °C-0 h or 800 °C-0 h had 2.5 times larger mesopore volume and 1.9 times larger mesopore ratio than the one by 700 °C-0.5 h. The fractional order of lactone increased from 700 °C-0.5 h (9.7%), 800 °C-0 h (10.6%) to 750 °C-0 h (24.5%). The fractional order of carboxyl groups increased from 750 °C-0 h (18.1%), 800 °C-0 h (23.0%) to 700 °C-0.5 h (31.4%). Activated carbon treated by 750 °C-0 h (227 F/g) and 800 °C-0 h (225 F/g) were higher than 700 °C-0.5 h (198 F/g). From these relationship, there would be no clear trend for specific capacity per weight under the situation having the nearly equal SBET. We assumed that mesopore ratio should be a main factor to obtain high specific capacity per weight at this moment.
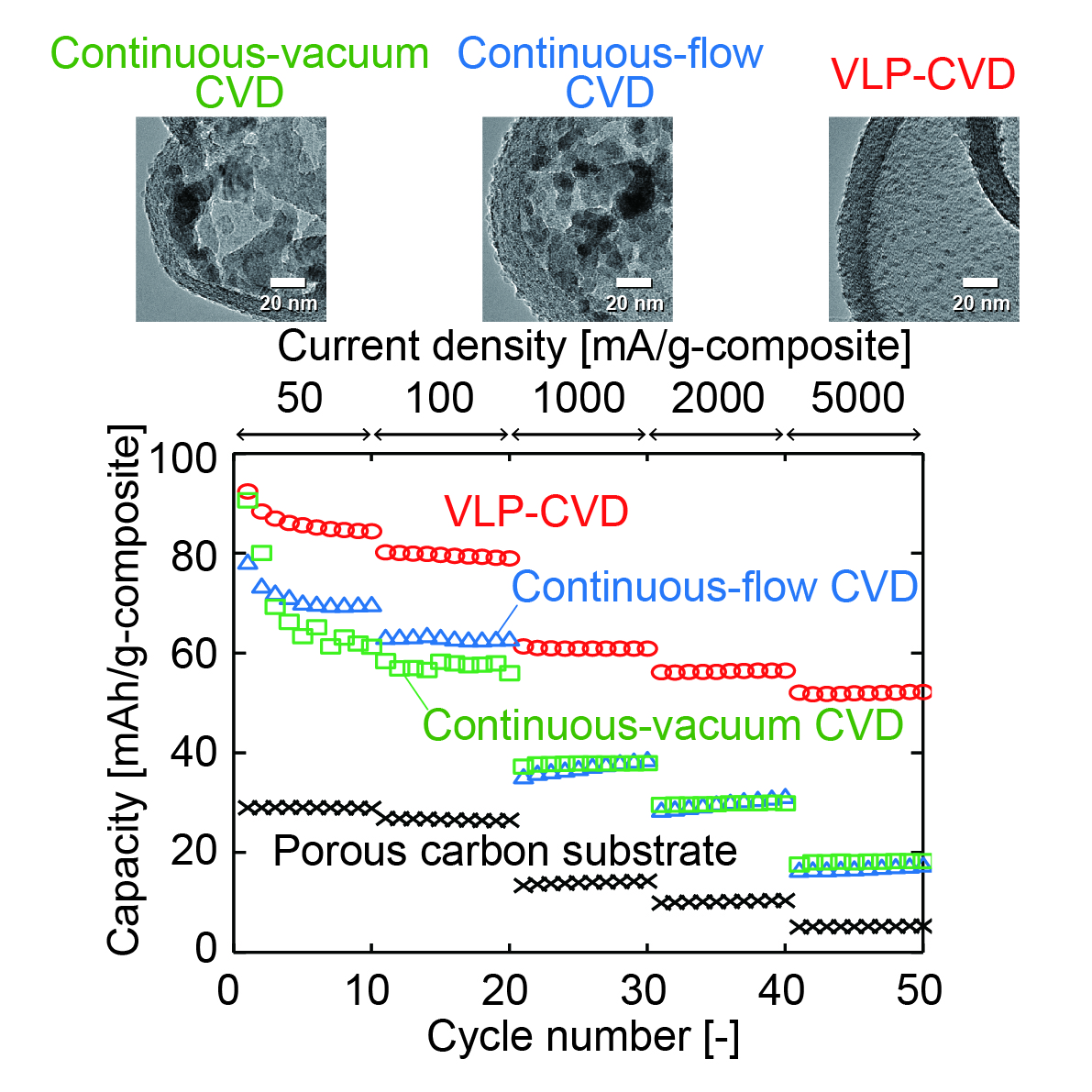
TiO2 is a promising material for the development of lithium-ion capacitors, because side reactions hardly occur at the charge/discharge potential of TiO2, compared to graphite. When using TiO2 as an electrode material, it is necessary to combine it with carbon at the nanometer level to improve its low electrical conductivity and low rate of reaction with Li+. However, preparation methods of reported TiO2/carbon nanocomposites are generally not cost effective, and their productivities are low. In this study, the vacuum liquid pulse chemical vapor deposition (VLP-CVD) technique was developed to easily prepare nanocomposites of TiO2 nanoparticles and commercially-available porous carbons. Using this technique, TiO2 nanoparticles with a diameter of ~4 nm could be homogeneously deposited inside pores of meso- or macroporous carbon. Because the deposited TiO2 nanoparticles connect to electrical conductive paths of the porous-carbon substrates, they showed a high discharge capacity of ~200 mAh/g-TiO2 (based on the TiO2 weight). In particular, the composite prepared from macroporous carbon showed extremely high rate performance, where 50% of the discharge capacity was retained at a current density of 15000 mA/g when compared to that measured at 50 mA/g. In addition, the composite also showed very high cyclability, where 80% of the discharge capacity was retained at the 10000th cycle. Because the VLP-CVD technique can be performed using simple apparatus and commercially available starting materials, it can be expected to apply this technique to industrial production of TiO2/porous-carbon nanocomposites for lithium-ion capacitors.
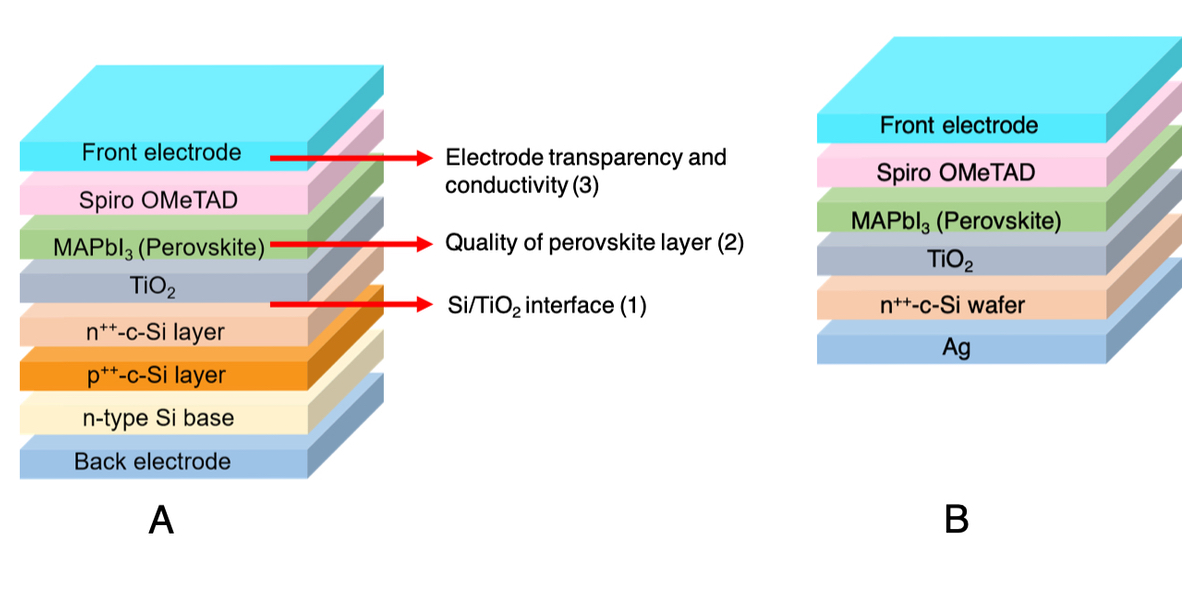
The utilization of crystalline silicon solar cell and organometal halide perovskite solar cell in tandem application has attracted a lot of attention for its high theoretical energy conversion efficiency (>35%). However, the approach of superposing these two solar cells raised issues that have to be clarified and rectified in order for this technology to reach its potential.
In a 2-terminal perovskite/Si solar cell configuration, the bottom silicon solar cell is superposed by perovskite based top cell using highly doped silicon layers as a tunnel junction. This approach comes with three main issues: 1. interface between silicon and TiO2, 2. quality of the perovskite thin layer, 3. transparency and conductivity of front electrode.
The main challenge in the Si/TiO2 interface is to reduce carrier recombination. In addition, constraints on fabrication techniques such as high temperature treatment in TiO2 deposition requires careful consideration to make sure a good surface passivating layer is fabricated properly. Secondly, as topological structure of substrate affects the structure of the thin film, the quality of perovskite layer when fabricated on silicon substrate and ITO covered glass has to be compared and analyzed. Thirdly, as light has to pass through the front electrode in order to reach perovskite absorbing layer, the transparency and conductivity of front electrode has to be optimized in order to achieve the maximum performance of the tandem solar cell.
In this study, we designed an experimental approach to understand these challenges by fabricating perovskite solar cell using highly doped n-type silicon wafer as substrate (Figure). We found that when typical TiO2 is used to passivate silicon wafer, the carrier lifetime decreased dramatically. To mitigate this phenomenon, we are currently modifying the structure of TiO2 with various annealing methods and analyzing the relationship between TiO2 structure and its properties as passivating layer.