
Exploring earth-abundant, active and stable electrocatalysts to replace noble metal materials for hydrogen evolution reaction through alkaline water electrolysis system is key to the development of sustainable energy conversion technologies. Here, we report a novel hybrid electrocatalyst comprised of atomically dispersed Ni-Nx species anchored porous carbon matrix with embedded Ni nanoparticles. Benefiting from the high surface area and strong coupling interaction of the Ni NP and Ni-N-C, the achieved Ni NP Ni-N-C EG hybrid electrode displays excellent electrocatalytic activity for HER in basic condition with a low overpotential of 147 mV to reach current density of 10 mA cm-2. The overpotential for the Ni NP Ni-N-C EG is well comparable to the best reported value in literature for all existing heteroatom doped nanocarbon catalysts and even lower than those reported for other transition metal based compounds in basic media. Furthermore, the Ni NP Ni-N-C EG hybrid electrode exhibits outstanding catalytic activity for overall water splitting under alkaline condition, as reflected by delivering a current density of 10 mA cm 2 at 1.58 V, which surpasses that of the benchmark combination catalyst for sufficiently high overpotentials. Electrochemical results, coupled with the thiocyanate poisoning experiments, HAADF-STEM analyses, XPS analysis, as well as the EXAFS and XANES results, reveal that the hybridization of atomically dispersed Ni-Nx active centers with that of embedding Ni NP modulates the electronic structure and facilitates electron transfer at the constructed interface, which synergistically boost the HER performance of Ni NP Ni-N-C. Theoretical calculations manifest that the incorporation of Ni NP into atomically dispersed Ni-N-C frameworks can effectively promote initial water dissociation process and simultaneously optimize the OH adsorption free energy on the Ni NP Ni-N-C, resulting in the improved kinetics of the HER in alkaline solutions.
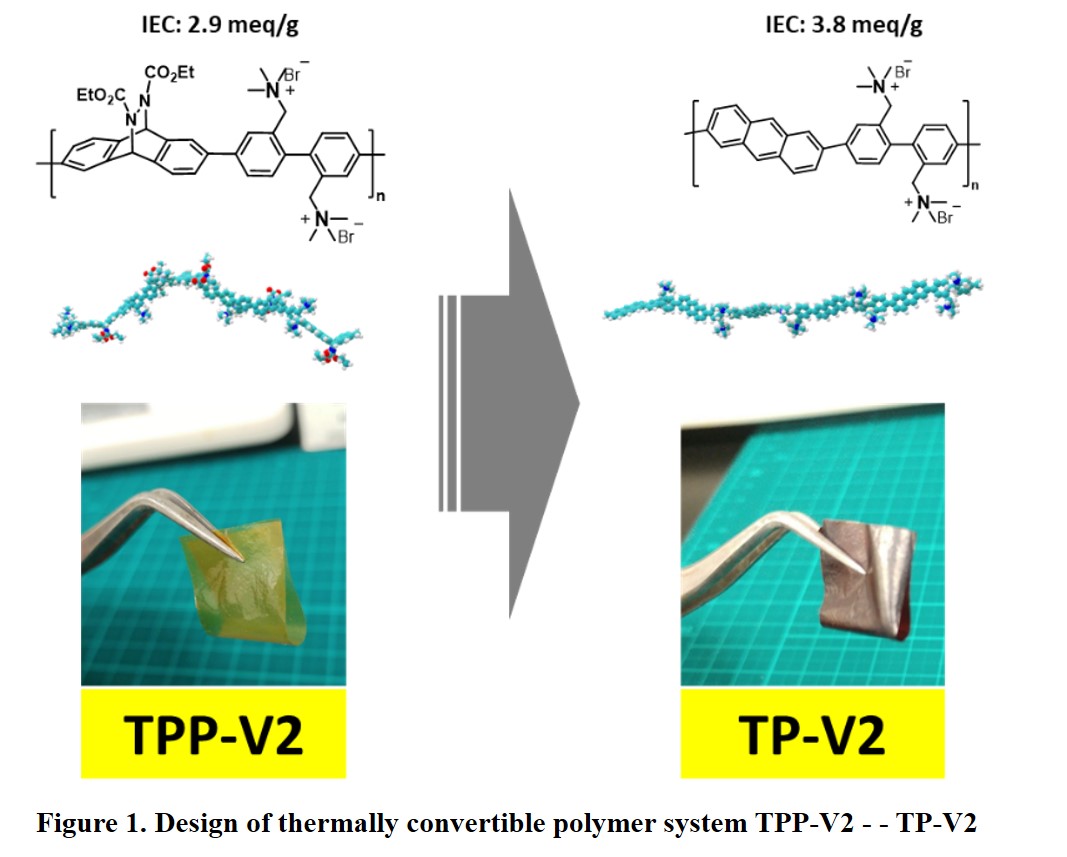
Hydrogen is a promising energy carrier to utilize renewable energy sources. It can be generated by using water electrolysis technology. Alkaline water electrolysis using anion exchange membranes (AEMs) draws a lot of attention. Besides being able to use non-noble metal catalysts, its performance is better than conventional alkaline water electrolysis thanks to lower internal cell resistance. However, the development of this technology is hindered by the lack of high performance and durable AEMs. Ether-free aromatic AEMs can be promising candidates to overcome the performance and durability issues. In this research, we proposed a unique AEMs molecular design using thermally convertible polymer system (Fig.1). Highly soluble precursor polymer is used to prepare a thin membrane. This precursor membrane then heated to obtain ether free backbone membrane.
We prepared the precursor polymer by Suzuki-Miyaura coupling of the monomers followed by bromination then quaternized to afford TPP-V2. Number average molecular weight (Mn), polydispersity (PDI) and ion exchange capacity (IEC) of TPP-V2 are Mn= 24,600 g/mol,, PDI=2.9 and IEC= 2.90 meq/g, respectively. Ultra-thin flexible membrane as thin as 8 μm can be cast from TPP-V2 solution in dimethylsulfoxide. TP-V2 membrane was obtained by heating TPP-V2 membrane at 180 oC for 1 h under vacuum. After conversion to TP-V2, IEC value increases to 3.78 meq/g. Despite higher IEC, water uptake of the TP-V2 is lower than it's precursor as π-π stacking appears. TP-V2 shows high alkaline and oxidative durability after exposed to 8M NaOH for 120 h at 80 oC (alkaline stability test) and 3% H2O2 & 3 ppm FeSO4 60 oC for 8 h (oxidative stability test). The ionic conductivity was almost the same even after exposure to such very harsh conditions. These results suggest our approach is promising for making high-performance AEM for alkaline water electrolysis application.
Acknowledgement: Part of this paper is based on results obtained from a project commissioned by the New Energy and Industrial Technology Development Organization (NEDO).
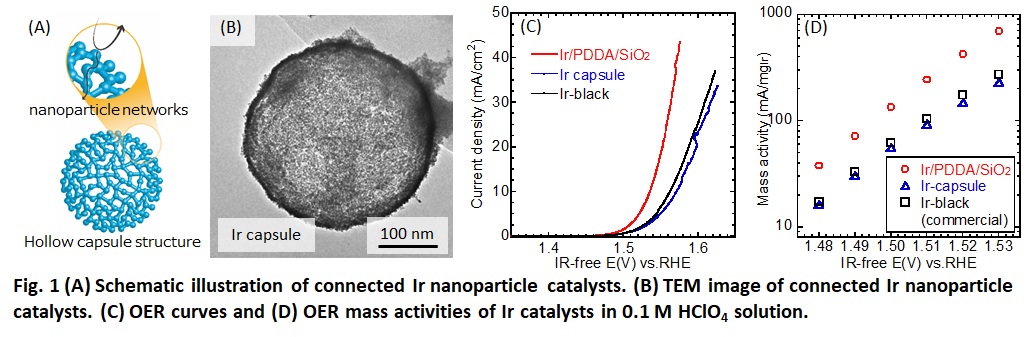
Oxygen evolution reaction (OER) catalysts for polymer electrolyte water electrolysis (PEWE) suffered from their low surface area because carbon support cannot be used for OER operating at high potential. Connecting metal nanoparticles enable catalysts to conduct electrons through nanoparticle networks without any support materials while keeping their high surface area. Thus, connected nanoparticle catalysts (Fig. 1 (A)) are promising for OER. Previously, we have developed connected Pt-Fe nanoparticle catalysts with hollow capsule structure for oxygen reduction reaction (ORR) in polymer electrolyte fuel cells (PEFCs).[1]
In this study, we proposed Ir nanoparticle catalysts for OER in water electrolysis because Ir has high OER activity. Ir nanoparticle catalysts were synthesized as follows. First, Ir nanoparticles were synthesized on silica template via polyol method using tetraethylene glycol as reducing agent and Ir(III) acetylacetonate as the metallic precursors (Ir/PDDA/SiO2). Then, these nanoparticles were coated with SiO2 using tetraethoxysilane in a mixed solvent of ethanol and NH3 solution. After coating, they treated in supercritical ethanol at 330 °C for 90 min. Dissolution of SiO2 in 3 M NaOH solution at 85 °C for 3 h made porous hollow structure (Ir capsule). OER performance were evaluated by cyclic voltammetry in 0.1 M HClO4 solution. A membrane electrode assembly (MEA) was fabricated by Ir/PDDA/SiO2 as an anode catalyst, and its water electrolysis performance was measured.
Fig. 1 (B) shows TEM image of Ir capsule. Capsule and networks structure of the catalyst were observed by TEM images. Fig. 1 (C) and (D) show OER curves and mass activities of Ir catalysts. Mass activity of Ir/PDDA/SiO2 was 2–3 times higher than Ir capsule and commercial Ir black. MEA using Ir/PDDA/SiO2 showed good performance.
[1] T. Tamaki, H. Kuroki, T. Yamaguchi et al., Energy Environ. Sci., 8, 3545-3549 (2015).
Acknowledgement: This paper is based on results obtained from a project commissioned by the New Energy and Industrial Technology Development Organization (NEDO).
Water electrolysis by using renewable energy such as wind and solar energy for hydrogen production is considered a promising way to storage the unstable electricity and realize low-carbon society. However, for large-scale application of water electrolysis, the key is to design low-cost electrocatalysts with high performance for the replacing of the noble metal based catalysts. Meanwhile, in the water electrolysis, the electrolyte includes acidic, alkaline, industrial waste water, and seawater with different pH values. It is expected to have the electrocatalysts which can work in a wide pH range, especially in the neutral solution so that it can be applied anywhere. Moreover, the solutions with the neutral pH value is harmless and environmentally friendly. In this study, nanostructured manganese-nickel mixed oxide with nanosheet array was fabricated on the Cu nanowires growning on the copper form <CF> by electrodeposition method, and then further phosphorized to MnNiP. The obtained Cu nanowire @ MnNiP/CF electrode was used for the hydrogen evolution reaction in water electrolysis. As a result, a low overpotential of 104 mV@10mAcm-2 with a small Tafel slope of 55.1 mV dec-1 in the neutral solution was achieved due to the enhanced charge transfer ability, improved surface active area and fast reaction kinetics.
Recent studies on polymer electrolyte fuel cell (PEFC) based energy generation have focused more on elevated temperature (above 100 °C), since high temperature PEFC (HT-PEFC) enhances tolerance to CO and prevents flooding as well as makes waste heat emission and its utilization easy. However, relative humidity (RH) management appears more difficult at elevated temperature. RH profile inside the cell should be well analyzed to ensure proton exchange membrane (PEM) is sufficiently hydrated to keep moisture content. In the current study, 1D model in the direction along the gas channel has been built for the straight co-current flow channels for analyzing water behavior at varied cell temperature and total pressure. Water permeation flux, moisture content, and proton conductivity of PEM were calculated. Employing straight channels, RH profile in the cell can be measured as change in RH at the cell outlet with varying the gas flow rate in experiments. Measured and calculated RH profiles through the gas channel were investigated at varied cell temperature. Total water flow rate at the outlet increases as the current increases. Partition of generated water to two sides is determined by the inlet RH and flow rate conditions. As the cell temperature is higher, the saturated vapor pressure is the higher. The more water generation is required to attain a certain RH through the cell at the higher cell temperature. This is the most important reason that water management is more difficult at elevated temperature.