
Lithium ion batteries (LiBs) are used in electric vehicles (EVs), hybrid electric vehicles (HEVs), compact devices and so on. However, both more power density and energy density is required, so active materials, binders, electrolytes, and separators are enthusiastically developed. Moreover, the ability of LiB determines not only materials but also the electrode structure. In the case of high output density cell, in other words, in the case of rapid charge or discharge condition, ionic conduction in porous electrode layer is dominant resistance, and it strongly depends on the heterogeneous porous structure. Recently, some researchers have been trying to understand the relationship between this porous structure and the effective ionic conductivity. In our previous research, the relative ionic conductivity was simulated in different porous electrode structure which consists of active material and binder, and the effect of morphology was discussed with electrochemical experiment, direct observation and numerical simulation. However, most of these research focused on only single component which is anode, cathode and separator. In order to increase the cell performance that is capacity, power density and durability, the effect of combination of these porous component have to be considered. In this study, various porous structure is modeled with direct observation results obtained by FIB-SEM and X-ray CT, and the charge and discharge simulation is carried out with some combination of these porous component. From this result, the morphology and the anisotropy of porous structure are evaluated to check the not only the internal resistance but also the ion flux distribution which cause to generate the Li dendrite. In addition, the effect of micro protective layer on ion flux distribution and conductive resistance is considered.
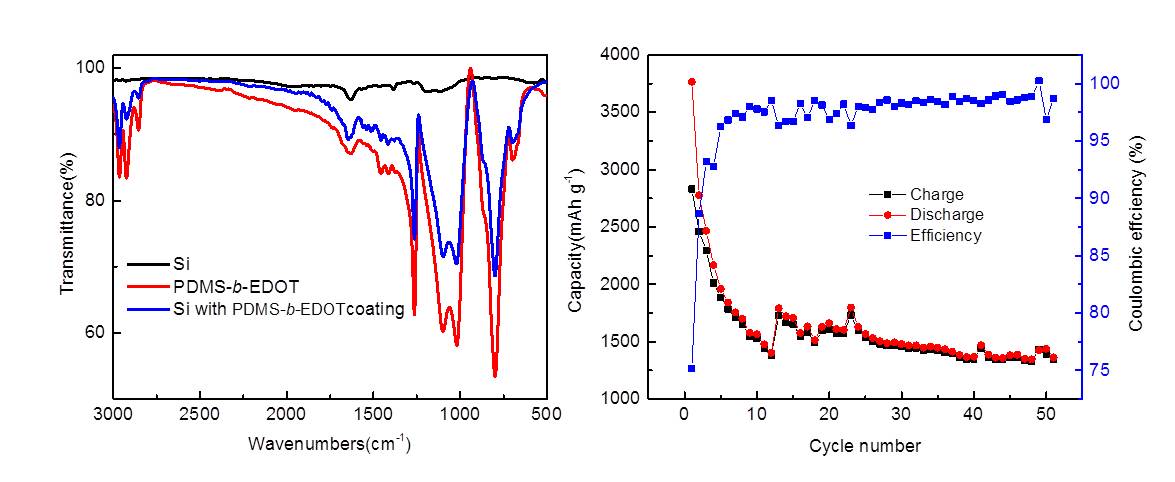
Silicon anode material has been considered as one of the most promising candidates for next-generation Li-ion batteries due to its high specific capacity. Its low conductivity and large volume change during charge-discharge process still hinder its practical applications. In this work, a conducting and flexible polymer is employed as a protective coating layer on the surface of commercially available silicon nanoparticles. The polymer can be prepared through hydrosilylation between hydride terminated poly(dimethylsiloxane)s (h2PDMS) and 3,4-Ethylenedioxythiophene (EDOT). The PDMS-b-EDOT was found successfully coated on the Si nanoparticles. The coated Si was found to show a much improved performance when it is used in making the anode for lithium ion battery. The cell assembled with a conventional PVDF binder shows reversible capacity of 1400 mAh g-1 after 50 cycles at the rate of 0.1C.
Acknowledgments: This work was supported by the Area of Excellence Grant from HKPolyU (1-ZE30), and the Science and Technology Foundation of Shenzhen (Grant No. JCYJ20170307150808594).
Lithium metal batteries <LMBs> are considered promising energy storage systems because of their high energy density and low redox potential. However, safety issue associated with the dendrite growth hinders their practical applications. One of the effective ways to suppress the dendrite growth and extend the cycle life is addition of the additive in the electrolyte. In this study, a novel low-cost electrolyte additive named as THP-Li salt was synthesized with a handy method for the first time, and introduced into both the solid-state electrolyte and the liquid electrolyte. For the solid-state electrolyte, the impedance value of solid polymer electrolyte <SPE> membrane was decreased apparently, and meanwhile, the all-solid-state LMBs with it delivered the excellent discharge capacity at high current density with good cycling performance <the capacity retention 78.7 % after 200 cycles> and higher coulombic efficiency. For the liquid ester-based and ether-based electrolytes, the addition of it greatly enhanced the overall cell performance of LMBs and particularly resulted in a higher discharge capacity, and remarkable improvement of the capacity retention <the capacity retention 68.4 % after 1000 cycles>. It is found that this novel additive can participate in the formation of stable solid electrolyte interphase <SEI> layer, preventing the side reaction between the electrolyte and electrode, suppressing the Li dendrites growth, which finally improved the safety and stability of LMBs.
A novel configuration by pressing two electrodes containing electrocatalysts for the oxygen reduction and evolution reactions (ORR and OER) into a bi-functional air electrode is designed for rechargeable Zn-air batteries. MOC/25BC carbon paper (MOC consisting of a-MnO2 and XC-72 carbon black) and Fe0.1Ni0.9CO2O4/Ti mesh on this air electrode mainly serve as the cathode of the ORR and the anode of the OER, respectively. Electrochemical studies include linear sweep voltammetry (LSV), rotating ring-disk electrode (RRDE) voltammetry, and the full-cell charge-discharge-cycling test. The discharge peak power density of a Zn-air battery with this unique air electrode reaches 88.8 mW cm-2 at 133.6 mA cm-2 and 0.66 V in the alkaline electrolyte under the ambient condition. After 100 discharge-charge cycles at 10 mA cm-2, an increase in 0.3 V between charge and discharge cell voltages is found. The long-time discharge-charge-cycling curve (10 h in each step) shows that the cell voltages of discharge (1.3 V) and charge (1.97 V) keep constant during the entire process. The performances of this rechargeable Zn-air battery are superior to most reports in recent literature.
Carbons in various forms are also chosen as substrates for uniform dispersion of a-MnO2 to form air electrode catalysts to evaluate the influences of carbon types on the catalytic activities of the ORR and OER (oxygen evolution reaction). The morphology and physicochemical properties of various a-MnO2/carbon composites are characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), and X-ray diffraction (XRD). Electrochemical studies include rotating ring-disk electrode (RRDE) voltammetry of catalysts, linear sweep voltammetry (LSV) of air electrodes, and the charge-discharge-cycling test of full cells. The discharge peak power density of Zn-air batteries varies from 66.3 (a-MnO2/carbon nanotubes with diameter ca. 10 nm, denoted as a-MnO2/CNT10) to 40.5 mW cm-2 (a-MnO2/super fine mesophase graphite powder) in 6 M KOH under ambient condition. The rechargeable Zn-air battery with the air electrode containing a-MnO2/CNT10 is stably operated for 100 cycles at 10 mA cm-2, which shows that an increase in 0.09 V between charge (decayed ca. 0.05 V) and discharge (decayed ca. 0.04 V) cell voltages.