
A deformable gel-packed chromatographic column was used to separate as-synthesized graphite oxide with different sizes. The synthesized gel (56 micrometer) was deformed by pressure of the fluid flow and the gaps in the gels showed a range of sizes. A suspension of graphene oxide (0.1 g/L, 10 mL) was injected, and graphene oxide in the elution had a size at 0.56 micrometer and 0.14 micrometer, whereas in half upper and bottom domain of the gel layer graphene oxide had a size at 33 micrometer and 2.9 micrometer, respectively, demonstrating that graphene oxide suspension was separated by size through gel layer. On behalf of graphene oxide, suspension of silica particle produced by dry process was also injected to the elastic gel -layer to separate silica particles due to their size and morphology.
To elute the filtered colloidal particle among the gel, the elastic gel layer was compacted and extended by the change of the applied pressure of water. At that time due to the dynamic change of the gel layer, the filtered particles was gradually eluted by the expansion of the gel's gaps as well as the convection of the fluid flow. The recovery percentage of the silica particles filtered was increased with increasing the repeated time of compaction and extension of the elastic gel layer.
The capture of solute into freezing part is difficult by the vigorous agitation of the freezing interface during the fast freezing of solution. We have been studying the applicability of ultrasonic irradiation (frequency: 20 kHz) to the agitating method, and found that the freeze concentration efficiency of solutes is improved greatly by this irradiation. In this paper, the effect of the frequency of ultrasonic irradiation on the freeze concentration characteristics for multiple solutes is examined.
Using three kind of solutions containing only one solute (Histidine, Vitamin C, and saccharose (these can widely be found in the food materials), 0.03 mol/L), we examined the freeze concentration characteristics with ultrasonic irradiation. The frequencies of ultrasonic irradiation were 20 kHz and 200 kHz, which were adjusted to the same output (11.8 W). From the experimental results, decreasing dissolved oxygen concentration (DO) can increase the concentration efficiency of all solutes in the case of 20 kHz ultrasonic frequency. On the other hand, in the case of 200 kHz ultrasonic frequency, increasing DO may increase the concentration efficiency. The DO dependence of the concentration efficiency differs depending on the frequency of ultrasonic irradiation and solute type.
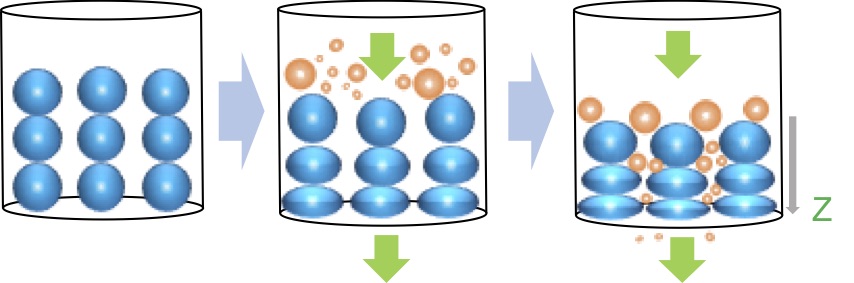
Ultrasonic wave is applied in many situations, for example cleaning glasses, sensors, dispersion. In cleaning glasses, the frequency of ultrasonic is about 100 kHz. When the frequency of sonication increases to about 2 MHz and vibrates water, water becomes mist and flies away. This phenomenon is ultrasonic atomization. In this research, we applied this atomization to drying particles that is useful in chemical processes. This drying method is called ultrasonic drying.
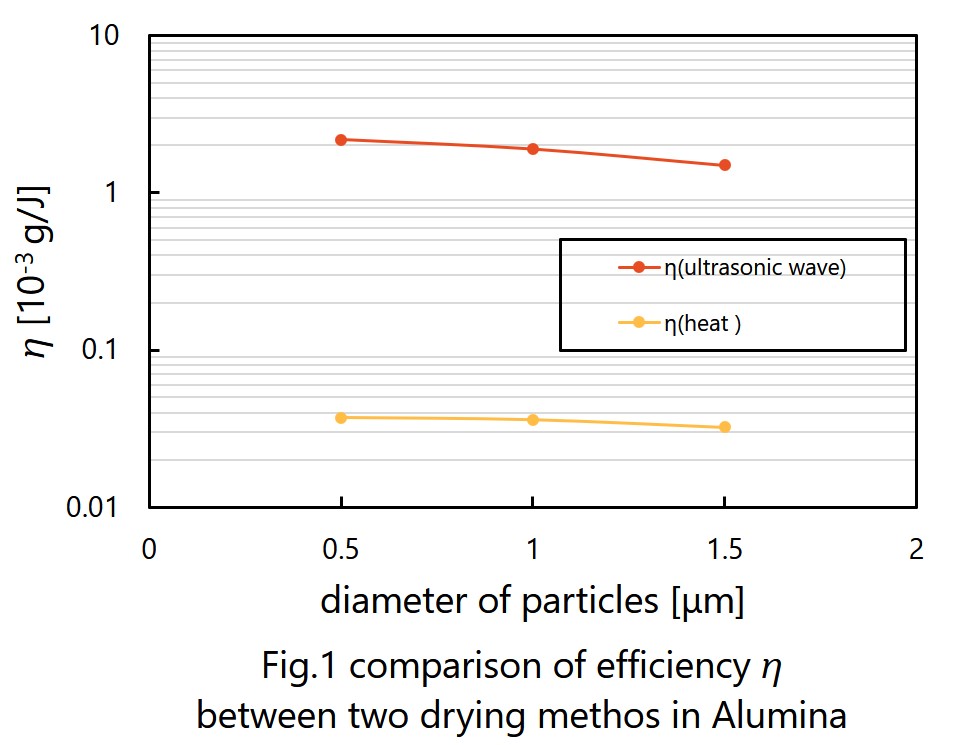
It is expected that ultrasonic drying will be faster and consume less energy than drying by heat. We had three experiments to prove them. First was testing whether ultrasonic can dry wet particles. I tried to dry fly ash by ultrasonic and observed how humidity of fly ash changed. Next is measuring the rate of ultrasonic drying. We prepared three kinds of particles, alumina, silica and fly ash. I moreover prepared three different diameter of alumina and two different diameter of silica to see whether the rate of ultrasonic drying depends on the diameter of particles. Last is comparing efficiencies of ultrasonic drying and traditional drying. We measured the loss of weight of materials by drying and the consumption of electronic energy. Efficiency of drying, as shown in Fig. 1, is defined by the weight loss divided by the electric energy consumption.
The result of first experiment was that mist appeared from the surface of particles, so we were sure that ultrasonic can dry materials. Second experiment suggests that the rate of ultrasonic drying has positive dependence on diameter of particles. This experiment, however, cannot show difference in dying rate among different particles. Efficiency of ultrasonic drying gotten in last experiment is approximately 100 times better than one of drying by heat. Considering by those outcomes, ultrasonic drying will replace some current drying methods in a few decades.
Consumer products largely originate from fossil resources which will be depleted sooner or later and contribute to CO2 emissions and climate change. Alternatives are sought with low carbon emissions and these are inexhaustible resources like plant derived biomass. Our motivation is to optimize the extraction process of lignin. The only water pre-treatment process and combination with organic solvent Soxhletation was carried out in the first stage to reduce impurities. Soda process and diluted acid process were chosen for extraction methods and microwave-assisted as the main instrument for heating up solution.
The results showed that pre-treatment process by using water following with isopropanol-hexane was given the best result. Water helped to extract sugar, alcohol, and some water soluble impurities. When isopropanol extracted the rest of polar impurities that water could not be done, Hexane was extracted non-polar impurities such as lipid. It could have extracted about 5.3 % weight loss of impurities without changed the lignin structure significantly due to TGA and FTIR results. This study aims to extract lignin in moderate temperature, pressure, and power in as little concentration and time as we could achieve. The process was done with soda process for 2 wt% NaOH concentration at 130 oC with 30-min extraction process as the best result rather than diluted acid process. The amount of lignin obtained about from this process was relatively higher both in 99.7% yield and 94.6% purity compared to other studies result. This type of lignin has similar characteristic to commercial dealkaline lignin.
This study aimed the treatment of the aqueous solution contaminated by antibiotics with duckweed, Lemna minor, which was reported to uptake organic compounds in the aqueous solution, and the removal of the antibiotics in the aqueous solution with duckweed under various conditions was measured to study the mechanism of antibiotics removal from contaminated solution. Firstly, it was confirmed that the molar concentrations of ciprofloxacin and sulfamethoxazole, which were selected as model antibiotics to be treated because of most popular antibiotics causing aquatic environmental pollution, could be reduced due to the mechanisms of hydrolysis, photo-degradation and uptake by duckweed. Ciprofloxacin was more degradable due to hydrolysis and photo-degradation in the aqueous solution than sulfamethoxazole, and the degrees of the molar concentration reduction due to uptake by duckweed were comparable for both antibiotics. In the cases of the treatment of ciprofloxacin of the initial molar concentration at 1×10-5mol/L, the reduction of the molar concentration due to the hydrolysis and photo-degradation shared 0.6 relative to the total reduction of the molar concentration, and the ratio of reduction due to uptake relative to the total reduction was 0.4. On the other hand, the molar concentration reduction of sulfamethoxazole due to the uptake relative to the total concentration reduction was 0.95, and the contribution of the hydrolysis and photo-degradation to the total reduction of ciprofloxacin concentration was so small. When the initial molar concentrations of both antibiotics increased up to 5×10-5mol/L, the contributions of these three factors to the antibiotics reduction were similar to the cases with the initial concentration as 1×10-5mol/L. Accordingly the uptakes of both antibiotics by duckweed were so influential to reduce the molar concentrations in the aqueous solution, especially for sulfamethoxazole, and this treatment method might have a potential to remediate the aquatic environment polluted by antibiotics.
The response surface methodology was applied to study and to optimize the surfactant-enhanced extraction of tea tree oil (TTO), i.e. the essential oil of Melaleuca alternifolia, by hydrodistillation method. Both Tween 20 and Tween 80 were used as surfactants added in the hydrodistillation vat with an aim to enhance the extraction yield of TTO. In principle, this study evaluated the relevance of several independent parameters, including the concentration of surfactant, extraction time, and liquid/solid ratio, against TTO yield with a design of experiment (DOE) based on response surface methodology. Central composite design (CCD) was used to optimize the processing condition of TTO extraction. The chemical compositions of tea tree oil were analyzed and quantized by GC-FID and were referred with the international standard "ISO 4730". Additionally, TTO obtained from optimal condition was examined on the stability of its microemulsion formulations as well as the antibacterial property, compared with the commercial TTO. The microemulsion stability was mainly determined by the particle size measurement with dynamic light scattering (DLS), whereas the antibacterial assay was carried out by agar disk diffusion method with Escherichia coli and Staphylococus aureus.
Keywords: Melaleuca alternifolia; Extraction; Design of experiment (DOE); Optimization