Solvent extraction (SX) is generally used for separation and purification of platinum group metal (PGM) in hydrometallurgical processes. The development of a successful SX process depends on the choice of appropriate extractants. Therefore, we have been studying the extraction behavior of PGM with newly synthesized extractants in addition to the structural properties of PGM complex anions extracted in the organic phase.
We have developed a new palladium extractant, thiodiglycolamide (TDGA), which can rapidly extract Pd(II) from HCl solution with a good selectivity and has a high oxidation resistance, compared with an industrial palladium extractant, di-n-hexyl sulfide (DHS). Currently, TDGA has been commercially available and already put to practical use in a PGM separation plant.
To date, there have been no effective practical extractants for rhodium in acidic chloride media, because the dominant rhodium species in relatively concentrated HCl solutions ([RhClx(H2O)6–x]3–x (x ≥ 4)) are poorly extracted into an organic phase. We have found that amide-containing tertiary amine (ACTA) compounds show a higher efficiency for the Rh(III) extraction from HCl solution than the conventional tertiary amine extractant, tri-n-octyl amine. The protonated ACTA molecules extract the mono-aquated dianion [RhCl5(H2O)]2– through the formation of an outer-sphere assembly, which was characterized by slope analysis, FT-IR, EXAFS, SANS, computational modeling, etc.
Prices of strategic metals and rare earth elements (REEs) have been rising over the past decade due to the global shortages in supply and increasing demands. These metals are essential components of advanced and emerging technologies associated with transport, environment, energy, defense, electronics, information and aerospace. With the utility of REEs projected to increase in the next decade, it is important to find alternative sources to conventional mining to cope with the demand. There are a few studies conducted on the potential of coal fly ashes as secondary resource of REE. Coal fly ash are waste products from coal burning in a coal power generation plants.
This research is aimed at extracting rare earth elements from coal fly ash by hydrochloric acid leaching. The purpose of this study is to explore the amenability of coal fly ash to metallurgical processing for the extraction of rare earth elements. Three process parameters were investigated in this study, namely, hydrochloric acid concentration, leaching time, and the leaching method. Results of the experiment showed that the recovery of rare earth elements increased with increasing hydrochloric acid concentration. Direct leaching method achieved higher recovery values for REEs compared to sequential leaching. Maximum recovery value for REEs was obtained at 1 h leaching time.
Scandium (Sc) is rare and expensive metal in high demand, providing excellent characteristics for various industrial applications. Since Sc is commonly found in crustal together with Y owing to its small ionic radius, the separation of Sc from yttrium (Y) and other rare earths is required. In the present work, separation and recovery of Sc in an aqueous chloride media was investigated by solvent extraction with carboxylic acid, Versatic acid 10.
The aqueous chloride solutions of rare earths were prepared by dissolving their oxides in 1 or 2 mol/L HCl solution. Organic solution was prepared by diluting Versatic acid 10 in IP Solvent 2835. Extraction of Sc and Y was carried out by shaking the organic and aqueous solutions at volume ratio of 1 : 1 at 25oC for more than 6 h. Concentrations of the metals in the aqueous solutions were determined by ICP-AES and those in the organic solutions were calculated based on material balance.
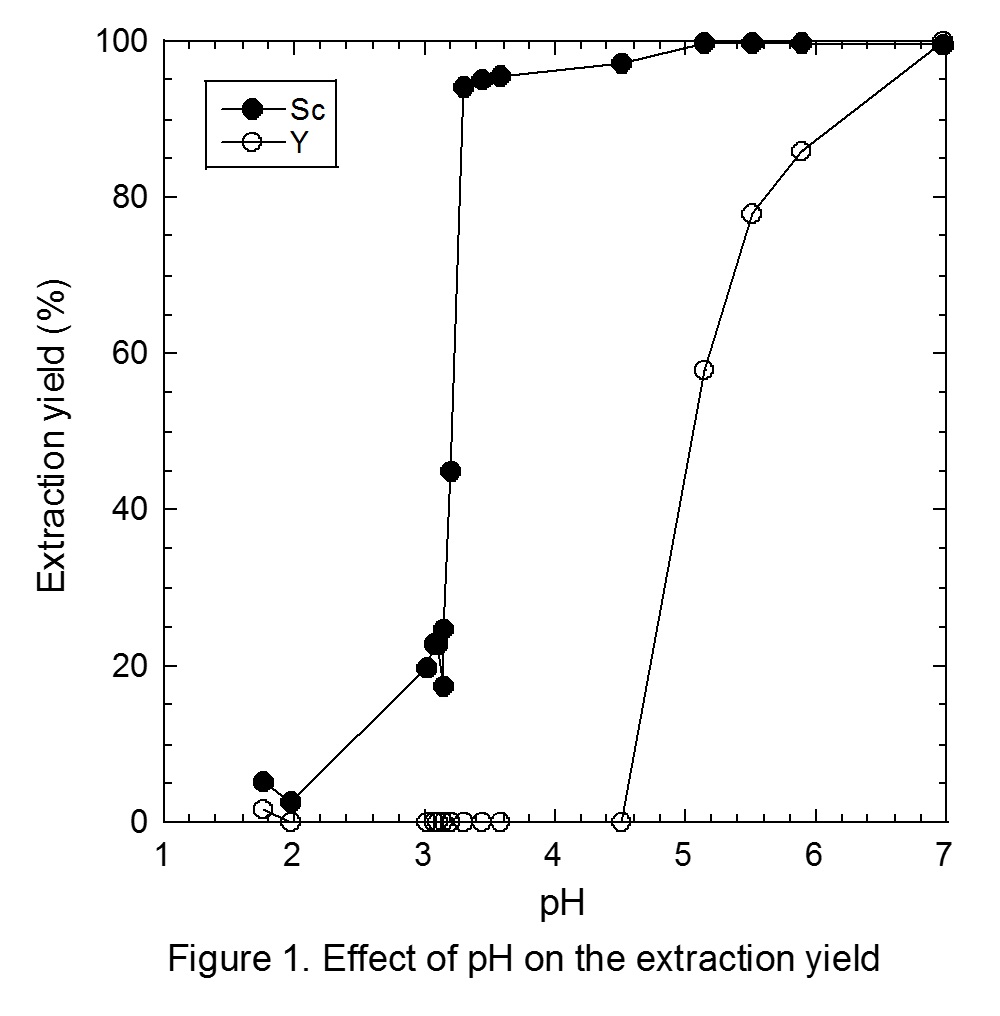
Figure 1 shows the effect of pH on the extraction yields of Sc and Y from the binary solution. Extraction of Sc proceeds from pH 3, and is increased with pH, while the extraction of Y proceeds from pH 4.5. Separation of Sc and Y is therefore easily achieved with Versatic acid 10. The conventional slope analysis of Sc with Versatic acid 10 revealed that the extraction is based on the cation exchange mechanism and the stoichiometry of Sc and the dimeric extractant of Versatic Acid 10 was 1 : 3.
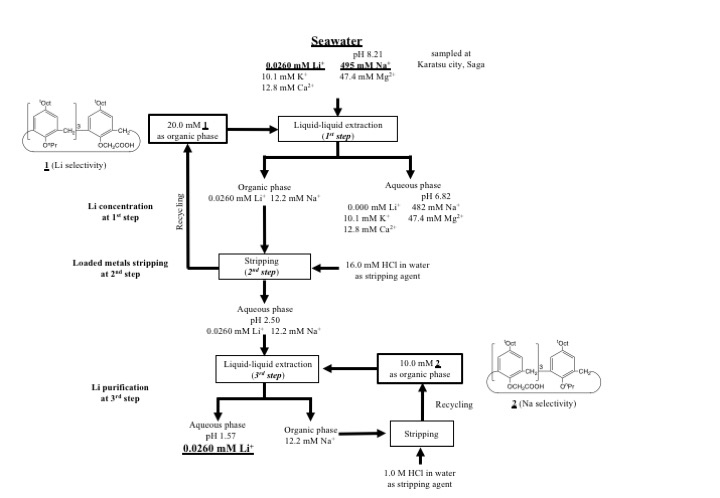
Two derivatives of p-t-octylcalix[4]arene were prepared to investigate extraction behavior of alkali metal ions. Tripropyl-monoacetic acid derivative (1) exhibited lithium selectivity among alkali metal ions, while Triacetic acid-monopropyl one (2) showed sodium selectivity. Then, they were employed for individual and stepwise recovery of alkali metal ions using microreactor system. Finally, they were employed for Li recovery from the seawater. Compound 1 was applied for Li recovery to concentrate Li ion at the 1st step and to strip the loaded metal ions, and compound 2 was employed to remove sodium ion for Li purification. Lithium ion was successfully completely recovered form seawater with two derivatives using microreactor system. Flow sheet for Li recovery with two derivatives using microreactor system is shown in Figure.