
Recently, the application of supercritical fluid, particularly supercritical carbon dioxide (scCO2), and polymer has been expected because it is safe and harmless for environment in increasing social interest in global environmental deterioration and rising costs of fossil fuels. For example, a polymer foam is one in which cells are dispersed inside a polymer, and the amount of material used can be reduced by the presence of the cells. Other examples include atomization and fiberization. Unfortunately, it is difficult to obtain sufficient products with only supercritical carbon dioxide and polymers. Therefore, attempts have been made to add a solvent as the third component. As a result, it is beginning to be reported that the performance can be changed more broadly than when no additive is added. The cause of this change is expected to be due to the change in physical properties by the addition of solvent, but the physical property data of the CO2/solvent/polymer ternary system are limited. Particularly, a phase behavior is important for the process design. In this study, the phase behavior of a CO2/toluene/polystyrene (PS) ternary system was measured.
The phase behavior was observed by an apparatus based on a synthetic method. In this system, two types of the phase boundary were observed; bubble point (BP) and cloud point (CP). The BP pressures were defined as the pressure at which small bubbles appear in the cell. The CP pressures were determined by measuring the turbidity of the system by the variation of reflected light intensity. In addition, the effects of composition, temperature, and polymer molecular weight for the phase behavior were examined.
Physical properties such as density, viscosity, and vapor-liquid equilibrium are indispensable information for the chemical process design. The demand for these physical properties is increasing in these days. In particular, the PVT relationship is one of the most basic and fundamental physical properties however, as the measurement needs specialized equipments and a long measurement period, the experimental data are always insufficient.
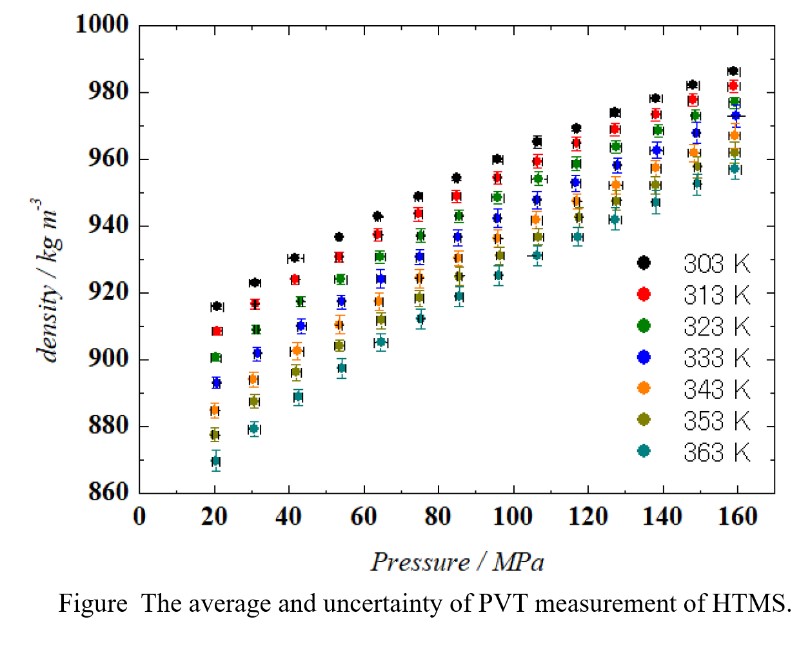
Silicon alkoxides are used extensively as raw materials of silica materials such as thin film, monolith, and silica aerogel which has high thermal insulation performance though, there are few reports on the thermodynamic properties of these substances. In this work, PVT relationships of hexyltrimethoxysilane(HTMS) was measured by an experimental apparatus based on a general variable volume method. Experiments were performed at temperatures from 303 to 363 K and pressures up to 160 MPa, and measurements were repeated at least three times.
Furthermore, from the above-mentioned subject in the measurement of physical properties, complementation of data by calculation as well as measurement is required. On this purpose, the equation of state (EoS) is a powerful tool. In this study, Sanchez-Lacombe Equation of state (SL EoS), whose parameters could be determined from only the PVT relationship. The measured PVT relationships of silicon alkoxides were correlated by SL EoS and the SL parameters were determined. In addition, estimation of the SL parameters of HTMS from the SL parameters of methyltrimethoxysilane (MTMS) reported previously was conducted with considering the molecular structure.
Methane hydrates (MH) in the Nankai Trough of Japan are considered as promising resources for developing CO2-free energy. In this work, the objectives were: (i) to develop an optical system for measuring method hydrate dissociation in situ, (ii) to measure change of occupancy ratio during MH dissociation and (iii) to develop a method for correlating occupancy ratio that can be used in large scale simulation systems. During the MH dissociation process, occupancy (θ) in each cage (θM or θS) changes based on degree of progress for dissociation. For analyzing the cage occupancy of MH, Raman spectroscopy was used as an in-situ technique to determine methane hydrate dissociation characteristics. MH films were synthesized with pure water and methane gas. The procedure used allowed MH films to form on the lower surface of the high-pressure optical cell sapphire window, which eliminated noise associated with the C-H stretching vibration of bulk methane gas. The changes in the relative cage occupancy ratio (3θM/θS) in the methane hydrates were monitored at equilibrium conditions and during changes of pressure in which dissociation occurred. The cage occupancy ratio (3θM/θS) in MH decreased with increasing pressure at equilibrium conditions due to increasing amounts of methane present in the S-cage. For an occupancy ratio (3θM/θS) of 3.37 (273.8 K, 5.0 MPa) the uncertainty in occupancy ratio obtained with procedures developed in this work was ca. 0.09 compared with a literature value of ca. 0.71. The Kihara potential parameters used in the model were redetermined to provide reliable correlation of the data. For depressurization conditions, (275.65 K, 7.60 MPa → 5.94 MPa), the cage occupancy ratio in MH increased up to 240 h monitoring time. The trend of the cage occupancy ratio with pressure and model will be useful for developing large-scale simulations of actual methane hydrates.
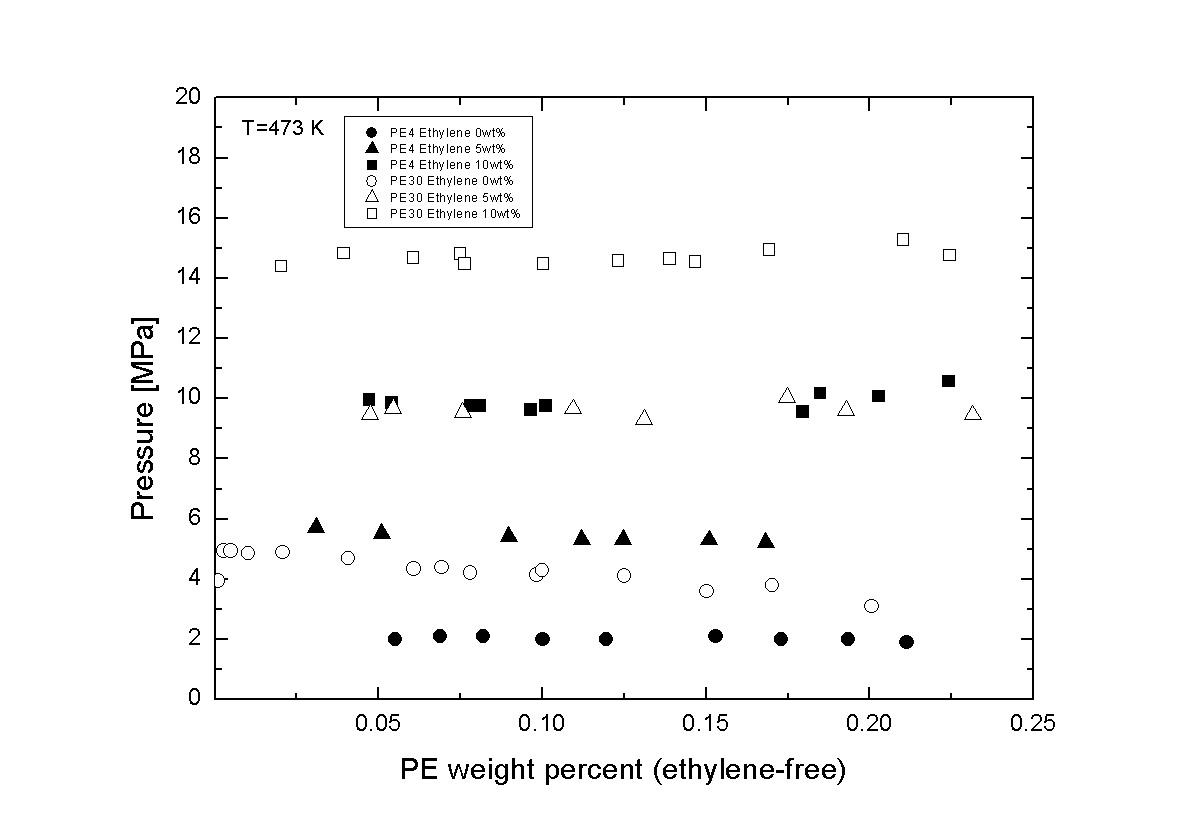
In recent years, several studies have reported the possibility of reducing energy consumption during the solution polymerization process of polyethylene by the introduction of a liquid-liquid (LL) separator. Besides temperature and concentrations, molecular weight (Mw) and polydispersity index (PI) are important factors for the design of the separation process. Unfortunately, the effect of Mw and PI to the phase behavior of PE + hexane + ethylene system has not been extensively studied. Previously, the LL phase behavior for PE + hexane + ethylene system was studied using polydisperse PE (PE30: Mw 30.0 kg/mol, PI 4.2) at 473 K. The addition of 1wt% of ethylene to the binary PE + hexane system increased the phase separation pressure by 1 MPa at almost the same magnitude over all feed PE weight fraction (wF,PE) range.
This study aims to provide experimental data and investigate the effect of Mw and PI to the phase behavior of PE + hexane + ethylene system. The experiments were conducted using polydisperse PE (PE4: Mw 4.0 kg/mol, PI 2.4) at 473 K with wF,PE ranging from 0.02 to 0.20 using a variable-volume optical cell equipped with a hand pump and a recording system. Comparing with PE30, the LL phase separation pressure of PE + hexane system for PE4 was about 3.0 MPa lower, and the addition of 1wt% of ethylene only increased the phase separation pressure by 0.8 MPa. The phase separation pressure increased as the Mw increased, and the addition of ethylene shifted the LL phase boundary to higher pressure. The results provided from this study could complement previous understanding for the design of the separation process.
The author proposed a new activity coefficient model [Y. Iwai, Fluid Phase Equilibria 465 (2018) 24-33]. The new model was derived from Guggenheim quasi-chemical theory with Taylor series. The formula of the new model is similar with that of regular solution theory. However, new idea in the new model is the surface area parameters, which account for the number of interactions between molecules, were assumed to vary depending on partner molecules and the concentration of mixtures. The new model can describe an exceptionally high value or convex behavior of activity coefficient in the dilute region. The liquid-liquid equilibria for ternary systems and the vapor-liquid equilibria for constituent binary systems can be correlated well with the same parameter sets.
The following results will be shown in this presentation.
(1) The new model is applied to calculate the activity coefficients for the systems which are difficult to correlate by widely used models. The target systems are as follows.
The systems of activity coefficients show convex behavior.
The systems of activity coefficients sharply increase or decrease in dilution region.
The systems of partially miscible.
(2) The new model is expanded to a group contribution method. Some results of the group contribution method will be presented.