
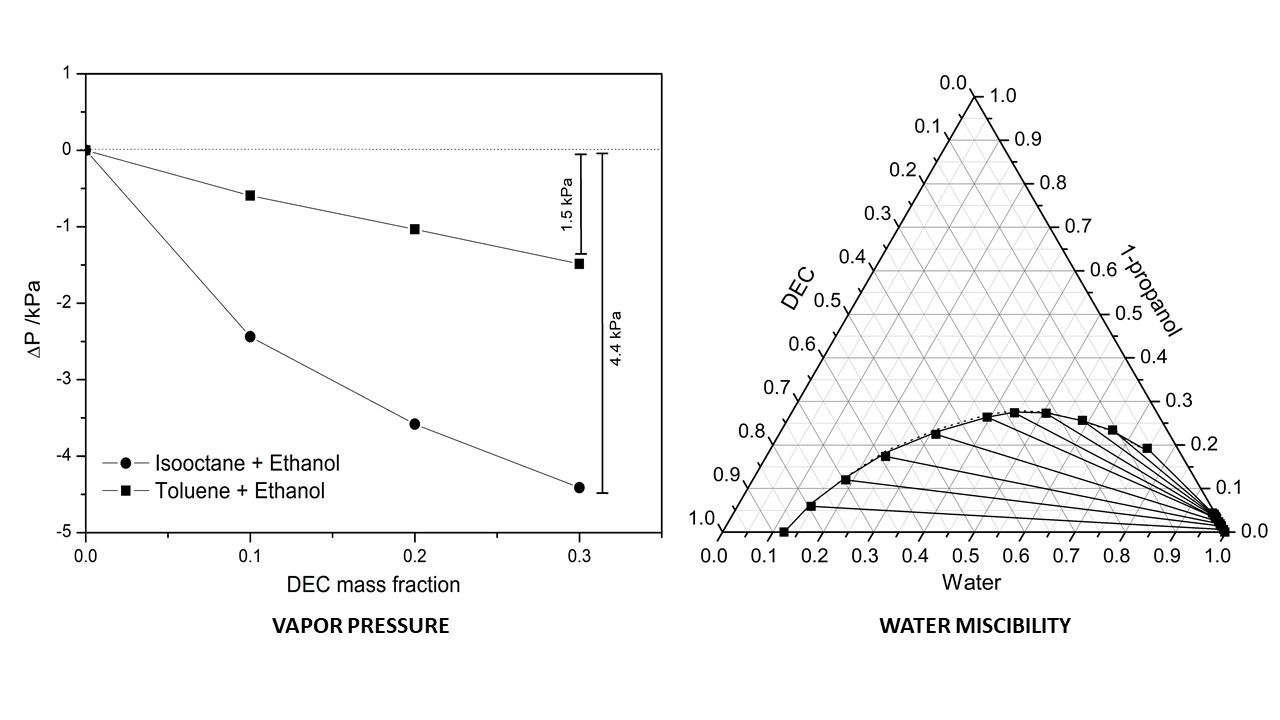
Diethyl carbonate (DEC) is one of the most promising compound that may be used as co-additive with ethanol/alcohols in gasoline blends because its higher octane number, lower vapor pressure and lower miscibility with water than ethanol. To apply DEC as gasoline additive, there are some aspects to be concern because blending process could change its physical properties such as vapor pressure and water miscibility. In order to know the physical properties of DEC-gasoline fuel mixture, the phase equilibria of DEC mixtures with several alcohols and hydrocarbons related to gasoline are required. Therefore, in this study, we reported the phase equilibria of DEC mixture with alcohols and hydrocarbons in binary and ternary systems at temperature range of 303.15 K – 323.15 K. From the isothermal vapor–liquid equilibrium (VLE) data, the vapor pressure of DEC mixtures with alcohols and hydrocarbons in binary and ternary systems were obtained. DEC is found to successfully decrease the vapor pressure in binary mixtures with hydrocarbons (isooctane, n-heptane, toluene) and alcohols (ethanol). In ternary mixtures, DEC is found to successfully decrease the vapor pressure of hydrocarbon + alcohol mixtures as well. The binary isothermal VLE data were well correlated using Wilson, nonrandom two-liquid (NRTL) and universal quasi-chemical (UNIQUAC) models with average absolute deviation (AAD) less than 2%. The binary parameters obtained from binary mixtures give good accuracy prediction results for ternary mixtures with AAD less than 5%. For the liquid-liquid equilibrium (LLE) containing of DEC and water, the water miscibility of DEC + alcohols + hydrocarbons mixtures were obtained. From the liquid-liquid equilibrium data of DEC + water + ethanol/ 1-propanol ternary mixtures, the presence of DEC in ethanol/ 1-propanol decrease the content of water. The LLE data for these ternary mixtures were well correlated using NRTL and UNIQUAC models with root mean square deviation less than 0.005.
When the critical properties of a pure substance are known, it is possible to predict thermodynamic properties based on the corresponding state principle using an equation of state. However, we can hardly find experimental values of critical properties of complex molecules such as ionic liquids. Extending the Joback method, I succeeded in predicting the critical temperature of complex molecules [1]. Moreover the critical volume of [hmim][Tf2N] was determined to fit the density of ionic liquid.
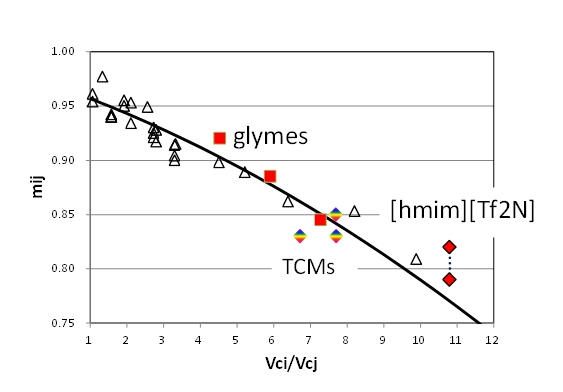
To calculate a mixture properties, especially phase equilibria, a binary interaction parameter, mij , should be determined. Changing mij values of glymes, TCMs and [hmim][Tf2N], the solubility of CO2 in the ionic liquids were best fitted.
In 1977, we reported that the optimal mij values for the modified generalized BWR equation of state were found to belong to several family groups expressed as a function of the ratio of critical volumes [2,3]. This time, recalculation brought a simpler correlation. As shown in a following figure for the CO2 containing family group, the above three kinds of ionic liquids were found to be on the same correlation curve. It means that polarity of complicated substances plays small role. It shows that the correlation of binary interaction parameter for the CO2-nonpolar substances can also apply to more complicated polar systems, such as ionic liquids.
[1] H. Nishiumi, Fluid Phase Equilibria, 420 (2016) 1–6
[2] G.H. Hudson, J.C. McCoubrey, Trans. Faraday Soc., 56 (1960) 761
[3] H. Nishiumi, S. Saito, J. Chem. Eng. Japan, 10 (1977) 176–180
Hydrogen reforming technologies, which combine hydrogen with toluene or naphthalene and store/transport it as methylcyclohexane or decalin, are expected to be widely used in the future due to the ability to safely transport the fuel with the highest concentration of hydrogen possible.
A reference database of equations of state is essential for the optimal selection of substances and the development of international technical standards. To add to this database, we started the development of highly accurate equations of state for these organic substances and their mixtures. We adopted the Helmholtz-type functional form, as with all other international standard equations of state for water and alternative refrigerants.
Throughout the fitting process, we used three types of input information for correlation. The first is the appropriate ranges of values of exponents and coefficients in the equations of state, the second is the numerical information on the shape of the thermodynamic state surface, and the third is the published measurement information for each substances. The present fitting program, which was developed by Lemmon [1], includes these three elements in the objective function effectively, and therefore we carefully controlled the behavior of each compound while simultaneously fitting the experimental data.
In our presentation, we will report the current status on the development of the equations of state being developed for the pure components used in the aforementioned hydrogen reforming technologies.
[1] E.W. Lemmon, “Equation of State Fitting with the REFPROP Program”, National Institute of Standards and Technology, Boulder, CO USA (2019).
In the past few years, the use of the combination between zwitterionic ion chromatography (ZIC) and the interaction liquid chromatography (HILIC) [ZIC–HILIC] in identifying specific substances has been developed and considered to be the best technique in identifying certain substances in a solution. A zwitterionic betaine polymer is known to be able to convey whole neutral charge since it contains both anionic and cationic active groups which are sulfonate and quaternary ammonium groups in the same polymeric repeat unit. This study was conducted aiming to develop gel with the characteristic of reversible thermosensitive in adsorbing heavy metal ions from its solution. There are three gels used in this study to adsorb heavy metal ion from salt solution provided Zn(NO3)2; copolymer gel consisting of zwitterionic betaine N,N-dimethyl(acrylamidopropyl)ammonium propane sulfonate (DMAAPS), N,N-dimethyl(acrylamidopropyl)ammonium butane sulfonate (DMAABS), and DMAAPS copolymerized with thermosensitive N-isopropylacrylamide (NIPAM) gels. These gels were used to investigate the adsorption ability and its swelling degree which was synthesized by free radical polymerization before being affected by methylene spacer number, copolymerization, and temperature given. This research found that as the temperature increases, the ability of the gel in adsorbing the ions decreases. In the case of DMAAPS and DMAABS gel, the swelling degree value increases when the temperature also increases. Meanwhile, sulfobetaine which has larger spacer has higher adsorption ability but not with its swelling degree. In spite of having more spacer than DMAAPS, DMAABS found to have the smallest swelling degree value. In addition, NIPAM-co-DMAAPS was found to have both the highest adsorption ability and swelling degree value. Eventhough copolymer has less amount of sulfobetaine than DMAAPS and DMAABS gels', the copolymer gel was found to have higher ability of adsorption as many as ten times of other gels.
Polyvinyl alcohol (PVOH) is widely used in many applications due to its biodegradable properties. Cellulose and MMT are the most common organic and inorganic material used as reinforcing filler. Kenaf is one of the candidates of natural nanocellulose. Three compounds (PVOH-kenaf nanowhisker-MMT) were blended, cast and analysed. Kenaf nanowhisker loading was varied from 0 phr to 8 phr while MMT loading was varied from 1 phr to 5 phr. Mechanical properties, thermal properties and microstructure were analysed. In overall, the tensile strength exhibited highest value at 5 phr of MMT and modulus exhibited the highest value at 3 phr of MMT loading although increasing MMT loading could reduce the elongation at break. Increment of kenaf nanowhisker loading would increase the tensile strength, elongation and modulus until a maximum point where beyond the mechanical properties decreased. Among all, nanocomposites with 5 phr of MMT with no addition of kenaf nanowhisker had the highest tensile strength.