
Poly(ethylene-co-vinyl acetate) (EVA) has been used as a commodity material because of the controllability of its properties such as rubber elasticity, adhesiveness, or transparency by changing comonomer vinyl acetate (VA) content in EVA. Since EVA polymers are commercially produced by a solution polymerization method in EVA solution including monomer VA and diluent alcohol, phase equilibria of the EVA solutions at EVA production conditions are important.
This study has conducted the measurements of the phase equilibria for ethylene + ethanol + VA + EVA quaternary system at temperatures from 313 K to 403 K and at pressures up to 25 MPa. In the measurement, the EVA composition in the solution was kept at 10 wt% while ethanol/VA ratio was changed. Vapor-liquid equilibria were observed in lower pressure region and liquid-liquid equilibria appeared in higher ethylene concentration. It was also observed that increasing ethanol/VA ratio causes bubble point pressure (BP) increase and cloud point pressure (CP) decrease at higher temperatures. In addition, experimental results were compared with ethylene + methanol + VA + EVA quaternary systems [1]. It was found that BP and CP for ethanol systems were lower than that of methanol systems.
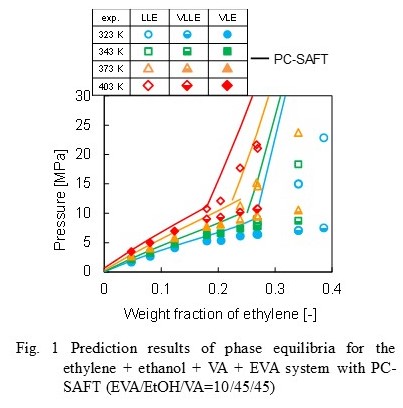
PC-SAFT [2] was applied to represent the phase equilibria of the quaternary system. Vapor-liquid equilibria of each binary system were correlated with the PC-SAFT to determine binary interaction parameters. Prediction results of phase equilibria for the quaternary system are shown in Fig. 1, showing relatively good results for PB but shift to higher pressures for CP at given composition.
[1] T. Nakamura et al., Fluid Phase Equilibria, 429 (2016)98.
[2] F. Tumakaka, G. Sadowski, Fluid Phase Equilibria, 217(2004) 233.
Deep eutectic solvents (DESs) are now widely acknowledged as a new class of solvents like ionic liquids (ILs) because they share many characteristics and properties with ILs. DESs generally have low vapor pressure and non-flammability, and their physicochemical properties are optimized. Then, DESs can be conveniently prepared by simply mixing a hydrogen bond donor (HBD) and acceptor (HBA) with a suitable composition. CO2 solubilities in DESs as reported in literature have been limited to hydrophilic ones and, to the best of our knowledge, hydrophobic DESs have not been systematically studied as potential solvents for CO2 capture [1].
In the present study, we have investigated the density, viscosity, and CO2 absorption behaviour of deep eutectic solvents (DESs), composed of halogenated onium salt (tetrabutylphosphonium bromide) and carboxylic acids (hexanoic acid or octanoic acid) at a 1 : 3 molar ratio (TBAC3HA or TBAC3OA). The densities and viscosities were determined at temperatures from 273.15 to 363.15 K and at atmospheric pressure. The CO2 solubilities in DESs and their saturated densities were measured at 313.15 K and pressures up to 8 MPa. The molarity of the CO2 solubilities were calculated from the experimental results. The experimental density was correlated by a quadratic equation. The viscosity data were represented fairly well by the Vogel-Fulcher-Tammann (VFT) equation. The CO2 solubilities increased with increasing pressure as observed for the typical physical absorption behaviour. The TBAC3HA and TBAC3OA gave higher CO2 solubilities than the ethylene glycol-based DES (TBAC3EG).
Reference
[1] L. F. Zubeir et al., J. Chem. Eng. Data, 63, 913-919 (2018).
Polymers have many excellent properties such as light-weight, electrical insulation and flexibility. Therefore, they are widely used as important parts in the industrial fields, especially, electronic industry. Recently, the thermal management inside the advanced electronic devices becomes more important because downsizing of the device proceeds with remaining high performances. However, thermal conductivities of polymers are generally quite low. Therefore, the addition of high thermal conductive fillers into the polymer matrix is often carried out to increase the thermal conductivity of the polymer materials. In addition to experimental works, theoretical studies are important to obtain the values in a wide range of the condition and to understand the fillers' configuration inside the polymer matrix. In the present study, the theoretical studies were carried out mainly using Hamilton-Crosser model to investigate the aggregation and orientation behaviors of the fillers. We focused on the hexagonal boron nitride/polyimide composites. By adjusting the configuration parameter in the Hamilton-Crosser model, the relationship between the effective thermal conductivity and possible configuration of the boron nitride particles was obtained.